
Project Category: Chemical
Join our presentation
Please feel free to join us via Zoom to ask us any questions about our project.
If the above button does not work please use the following link or use the meeting ID:
https://ucalgary.zoom.us/j/92604672474
Meeting ID: 926 0467 2474
About our project
The novel coronavirus pandemic has brought on a year of new health standards ranging from masks, more regular cleanings, and increased use in disinfectants. Our project aims to create a bio-surfactant based disinfectant that can be applied in the COVID-19 pandemic. Through the use of the bio-surfactants we aim to limit the potential environmental and health effects from the overuse of bleach based disinfectants and quaternary ammonium salts. Our objective is to have a 99.9% inactivation of virus activity over a 1 minute contact time with a secondary objective to have strong anti-microbial and anti-bacterial properties. To meet these requirements we have developed a disinfectant based on the bio-molecule surfactin.
The disinfectant is composed of 6 components: Surfactin, Ethanol, Hydrogen Peroxide, Citric Acid, Sodium stannate, Sodium Hydroxide, and Water.
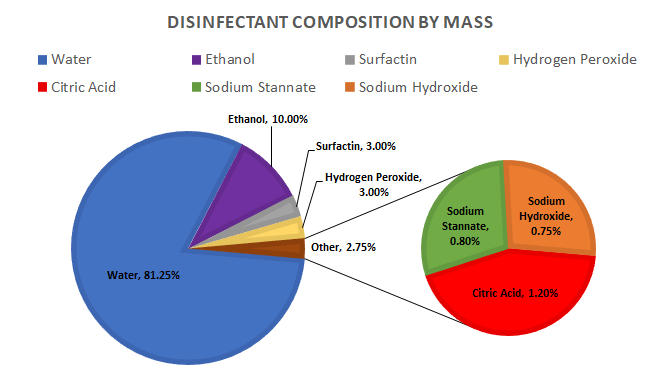
Surfactants inactivate viruses by using their amphiphilic properties to bind to the phospholipid bilayer of an enveloped virus. As the surfactant forms around the contaminant it will be lifted from the applied surface by the hydrophilic heads and taken into the solution. Once in solution the contaminant is suspended and will not be able to interact with it’s surroundings or infect thus making it inactive.
Through this mechanism and the anti-bacterial properties of other agents, such as hydrogen peroxide and ethanol, we aim to create an environmentally conscious product that is effective in both the COVID-19 pandemic and future pandemics.
What does each component do?
Each component plays an important role in the disinfectant for targeting different areas.
Ethanol
- Greater exposure for the active ingredients against target contaminants.
- Reduces surface tension.
- Helps stabilize the composition.
Hydrogen Peroxide
- Anti-bacterial / Anti-microbial properties.
- Acts as the secondary active component.
- Ensures disinfectant is well rounded.
Sodium Stannate
- Stabilizes for hydrogen peroxide.
- Increases shelf life for product.
Citric Acid
- Sequestering agent.
- Prevents precipitation of surfactin with metal ions.
- Assists with contamination control.
Sodium Hydroxide
- Buffer agent.
- Prevents precipitation of surfactin by controlling pH.
Surfactin
- Active agent.
- Ability to inactivate enveloped viruses.
- Can destroy biofilms.
Water
- Dilutes solution for safe use.
- Solvent for components.
Meet our team members
Contact us:
Dante Forcinito
Karim Firdous
James Pond
Walid Benchamma
Kyle Schofield

James Pond 
Kyle Schofield 
Walid Benchamma 
Karim Firdous 
Dante Forcinito
Details about our product
HOW OUR DESIGN ADDRESSES PRACTICAL ISSUES
As disinfection use has risen since the start of the pandemic the presence of bleach based disinfectants and quaternary ammonium compounds (QAC) have been observed in soil and wastewater. [2] Surfactants, such as QAC’s, will interact with a variety of biological components including bacteria and growths that are required to maintain a healthy ecosystem. To limit the impact from the overuse of disinfectants we must takes steps to limit the amount of cleaning products entering the environment as well as consider more eco-friendly compositions. The motivation for this project is to create an environmentally benign surfactant based disinfectant
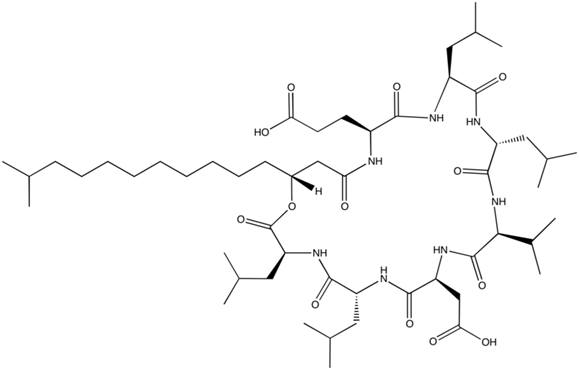
We selected a product of bacillus subtilius, surfactin (C-15) as our primary agent. The bio-surfactant has been previously suggested to be used in oil spill remediation as it is highly biodegradable. [3] Similarly, the surfactant is near harmless to people and requires a significant amount to be ingested before any risk of acute or chronic health problems. Figure 3 below is a visual model for surfactin.
WHAT MAKES OUR DESIGN INNOVATIVE
We are breaking away from tradition soaps, detergents, and disinfectants by employing bio-surfactants. Bio-surfactants are typically not used in disinfectants due to problems associated with storage and costs with cultivating the bacteria. By having our own method of producing a bio-surfactant we may overcome the associated problems and high costs of bio-surfactants in the market.
WHAT MAKES OUR DESIGN SOLUTION EFFECTIVE
Our design meets all of our design criteria. Our formulation provides broad spectrum disinfection of hard surfaces within short exposure times. The disinfectant is easy and safe to use in residential settings. The production and life-cycle of the disinfectant is environmentally conscious and will be sustainable into the future. Our bioprocess for surfactin production also makes the disinfectant cost effective for an expanding market.
HOW WE VALIDATED OUR DESIGN SOLUTION
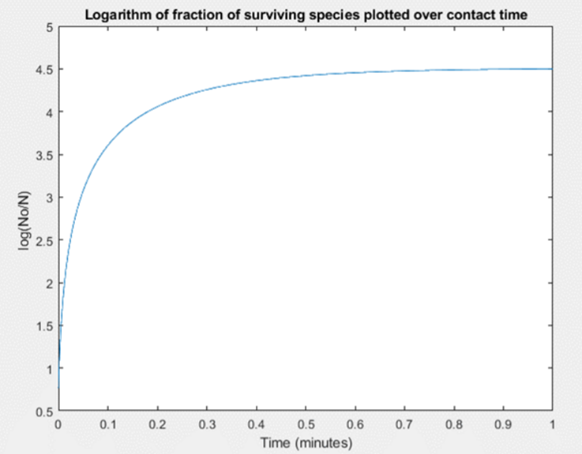
The efficacy for our design was first simulated in MATLAB using an intrinsic kinetic model for disinfection to verify our solution composition.
We were able to model the effect the initial concentration of surfactin (bulk) had on the inactivation of a virus titer. We found that at a 3% by weight composition of surfactin at least 99.9% of microbials (using an initial 1.4×108 titer) were inactivated. This 99.9% inactivation falls in line with the Canadian Food and Drug Act guidelines for a general use hard surface disinfectant. Figure 4 below is the plot for the disinfection kinetics.
Furthermore, the solution was tested in a lab against a sample of E coli to test the anti-bacterial properties of the solution. Figure 5 below provides an overview of the experiment. Two disinfectants were made, one contained the the active ingredient surfactin and the other did not. Both solutions demonstrated strong anti-bacterial properties reducing the colony-forming units (CFU) from 175 CFU to 0 visible CFU.
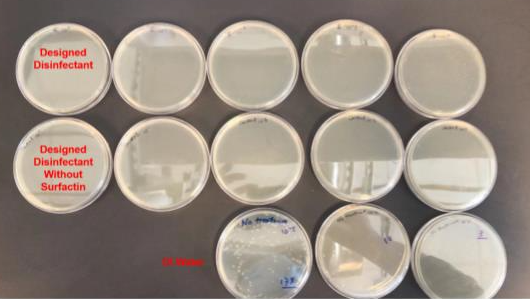
The hydrogen peroxide and ethanol both contribute to the anti-bacterial properties of our solution. Surfactin is there to target viruses, however it will have an effect against bacteria and biofilms which can be resistant against our secondary agents. This evidence supports our secondary goal of creating a general use disinfectant that is effective against a broad range of bacteria.
FEASIBILITY OF OUR DESIGN SOLUTION
Our product looks to sell 3 different products of disinfectant as units of 1L, 4L, and 120L which are sold at respective prices of $12, $34, and $950, where the 1 L and 4 L units would be sold commercially on the national level, and the 120 L would be sold internationally as a bulk product. Overall the process will be producing 2 million kg annual of the disinfectant with a total of 1.2 million units produced of which are split as 50% being 1 L, 40% being 4 L and 10% being 120 L. Assuming that all the product disinfectant would be sold we would look at an annual revenue of 20 million dollars with an annual expense of 10 million dollars meaning a net profit of 10 million per year. When evaluating the feasibility of our project a cash flow analysis was done over a 10 year period to see the longevity of the project which was found to initially require a total capital investment of 54 million dollars due to required processes to first produce the surfactin and then to produce the disinfectant solution, but the overall project was found to have a net present worth of 26 million dollars with a DCFRR of 13.7% followed by a payout period of 5 years.
As for the sensitivity of the project should still be feasible with a range of 20% decrease or increase in the 3 main factors of the fixed capital investment, expenses and revenue as the Net present worth remains positive, but larger degrees of variation are shown when revenue is changed compared to the expenses and FCI. This means that our product is both technically feasible and economically feasible.
ENVIRONMENTAL AND HEALTH RISKS
Health Risks
There are few health risks associated with the disinfectant, the most dangerous component is ethanol which can be carcinogenic over time. However, this would require significant and regular exposure and or ingestion. Chronic exposure should be considered when designing the process. Some associated health risks with the product are outlined below:
- Ethanol may result in eye or skin irritation and can be potentially toxic if ingested. Chronic exposure may result in cancer or nerve damage.
- Hydrogen Peroxide may cause skin and eye irritation, can be potentially toxic if ingested. Respiratory damage may be a result of extended exposure.
- Surfactin may be toxic if ingested in significantly large amounts.
- Citric Acid may cause numbness, muscle spasms, or stomach pain if ingested.
- Sodium Stannate is toxic if ingested. Chronical exposure may result in respiratory irritation or skin and eye damage.
Appropriate measures for waste storage must be considered. This includes sectioning off and storing organic waste with proper signage. Canadian legislation states that organic waste must be disinfected before transport to protect the public from any potential contamination. This means that large storage containers containing any waste product from the production of surfactin or the disinfectant will have to be cleaned before removal. The waste from this process is low threat, however proper disinfection of the waste containers must be carried out.
Environment
We have set our target for environmental protection to align with the Canadian Environmental Protections Act. As long as the product is not classified as a persistent chemical within the environment (half-life of182 days) then the project falls within our design requirements. As the concentrations of most components within the system are low we can analyze the consider the largest component the largest threat, in this case it is ethanol. However, literature states that ethanol only has a half-life of less than 2 days in water and soil, therefore the disinfectant should pose a low risk to health and safety of the environment.
Society
Societal pressure for cleaner products and processes grows as increased concern over climate change mounts. Safe and environmentally conscious products have previously been shown to be successful for disinfectants. One of our largest competitors to our proposed disinfectant is Eco Sanitizer based out of Vancouver, Canada. They have shown success in selling QAC based disinfectants with a revenue of 3.9 million in 2020. This demonstrates that there is market for products similar to what we produced.
Proposed Process
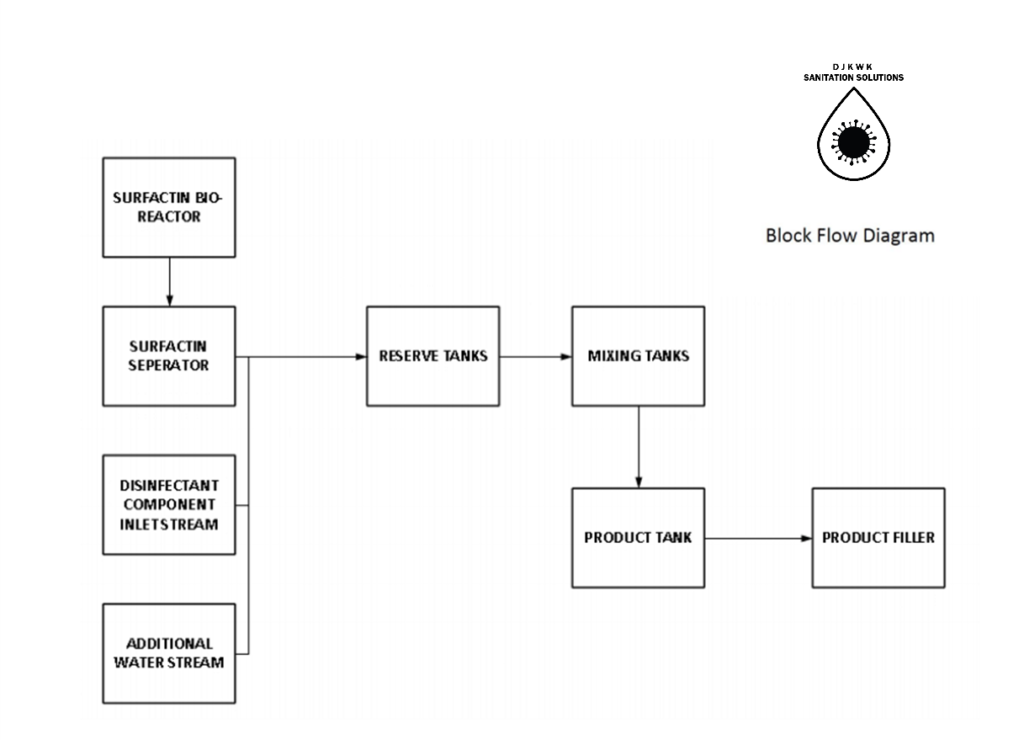
The overall process for creating this disinfectant consists of three major steps: First is the production of surfactin and the purification to prepare the surfactant for mixing. Next is the mixing step to combine all of the above components into a solution. The final step is a bottling process where the disinfectant will be placed into 1L or 4L bottles. The mixing and bottling operation time is over 16 hours a day with two 8 hours shifts in order to match surfactin rate of production. Figure 6 represents a block flow diagram of the process.
SURFACTIN PRODUCTION
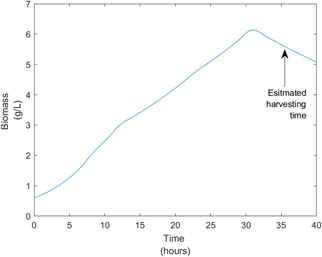
Producing our own Surfactin became a necessity as purchasing bulk amounts of Surfactin was not an option due to it’s high costs. When designing our bioprocess, we focused on controlling variances of key parameters such as pH, temperature and dissolved oxygen levels. This was crucial to achieve optimal yields. We also modeled the growth kinetics of our process using the Monod kinetics model, this helped us to learn more about our process and estimate a batch time of 34 hours. Figure 8 below provides a visual aid for this process.
SURFACTIN PURIFICATION
Once the bioreactor has finished its growth cycle, the harvested broth and excess foam is dissolved and passed through a membrane filtration process. The broth is passed through a series of polyether sulphone membranes of decreasing porosity, removing large cell debris in the first stage, and concentrating surfactin between the second and third stages by allowing smaller products to pass. The purified surfactin is then pumped to the mixing tanks already in a proper solution for use immediately.
MIXING
The entering feed into the mixing tank is thoroughly mixed at a blending power of 2.2 kW with five 100L mixers working continuously. The mixing is done over 1 hour intervals for each new batch of feed entering which allows for thorough mixing and adequate time for the exiting solution to leave with the desired composition. This thorough mixing ensures complete miscibility and that all components are dissolved into one solution. This is then fed into a product reserve tank to then be later bottled and shipped off.
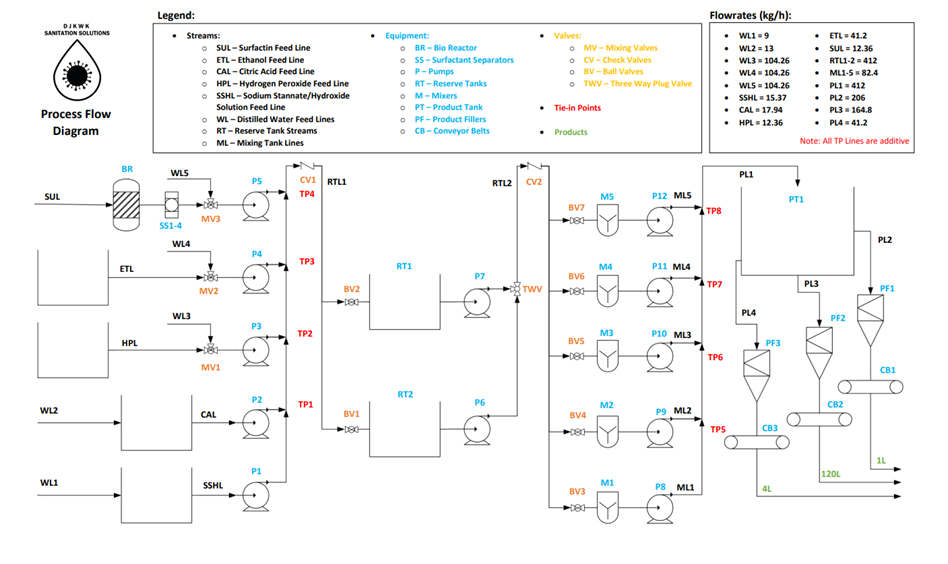
OVERALL PROCESS
Partners and mentors
We would like to thank everyone who assisted us and provided us with feedback in the development of this project. Our supervising professor Dr. Gemma Lu provided us valuable feedback as we progressed our design. Furthermore, Xiao He provided us with support and feedback for our composition and lab procedure as well as organizing the testing for the disinfectant.

References used on this page:
[1] L. Dorn, “How Washing With Soap Kills Viruses of All Kinds,” 2020. https://laughingsquid.com/how-washing-with-soap-kills-viruses/ (accessed Apr. 11, 2021). [2] P. I. Hora, S. G. Pati, P. J. McNamara, and W. A. Arnold, “Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications,” Environmental Science and Technology Letters, vol. 7, no. 9. American Chemical Society, pp. 622–631, Sep. 08, 2020, doi: 10.1021/acs.estlett.0c00437.
[3] J. Q. Feng, H. Z. Gang, D. S. Li, J. F. Liu, S. Z. Yang, and B. Z. Mu, “Characterization of biosurfactant lipopeptide and its performance evaluation for oil-spill remediation,” RSC Adv., vol. 9, no. 17, pp. 9629–9632, 2019, doi: 10.1039/C9RA01430F.
