PROJECT CATEGORY: CHEMICAL
Lifetime Recycling Ltd.
Join Our Presentation on Zoom
April 5th 2022, 10:00AM – 12:30PM
OUR PROJECT
Batteries are extensively used around the globe today. This ranges from common household items requiring single-use batteries, handheld electronic devices containing rechargeable internal batteries, and large-scale machinery and vehicles using high voltage cells. They are a staple of modern life, and demand is exponentially increasing as the globe transitions towards reducing destructive emissions.
Applications in numerous industries have led to rising pressure to ramp up manufacturing and production. This unprecedented growth is being inhibited due to excessive necessity of lithium and cobalt, which are currently the most common precursor metals used in the fabrication of cathode active materials.
Spent batteries are currently discarded once depleted and are sent straight to landfills. Here they contribute towards environmental degradation as heavy metals leak into the ground, mixing with soil and groundwater causing irreversible ecological damage. They also release toxic gasses through the common practice of incineration of landfill waste.

Li-FETIME Recycling has identified a unique solution to both of the aforementioned problems through the establishment of a hydrometallurgical process and operating plant. This plant located within Alberta will recycle waste lithium-ion batteries for the recovery of valuable materials including lithium and cobalt. Our design hopes to reduce the environmental impact of waste batteries and provide the resources for an electrical future.
Video Overview On Our Process
MEET THE TEAM





HIGH LEVEL OVERVIEW
HOW OUR DESIGN ADDRESSES PRACTICAL ISSUES
With countries around the globe scrambling to reduce emissions, and reach carbon neutrality within the next 20-30 years, there will be increasing demand for batteries across a variety of sectors such as the innovative electrical vehicle industry. Lithium and cobalt are currently the most common precursor elements used in the fabrication of electrodes for battery technology. Demand for these metals is expected to skyrocket by 2030, and current production rates are a fraction of anticipated industrial / consumer needs.
Both of these metals are currently difficult to extract and refine, and supply cannot easily be increased. Lithium metal must be electrochemically isolated from a mixture of potassium chloride and lithium chloride ocean brine which is a cost and energy intensive process. Meanwhile cobalt is buried deep below the earth’s crust entrenched in copper and nickel ore. These deposits must be mined and processed using reductive smelting of metallic-lustrous ores such as cobalite. This is where Li-FETIME Recycling offers a unique solution to this problem by recycling these valuable raw materials from discarded battery waste, providing 98% cobalt and 81% lithium recovery.
WHAT MAKES OUR DESIGN INNOVATIVE & EFFECTIVE
There is currently insufficient battery waste recycling infrastructure specifically within Alberta and to a lesser extent around Canada. In 2021 alone, it’s estimated that Canadians threw out over 745 million batteries which accounts for 41,000 tons of battery waste. This staggering amount compared to the reported recycling of just 4,100 tons of batteries translates into a meager 10% recycling rate.
Current major players such as Call2Recycle based in Ontario employ the pyrometallurgical process. This recycling operation only offers the recovery of cobalt and has numerous disadvantages associated from a plant scalability standpoint. The pyrometallurgical approach also requires extensively high energy consumption. This is due to battery preheating which occurs at 300℃ to melt plastics, and the pyrolysis itself which is controlled at over 700℃. These operations result in the release of toxic gasses such as dioxins, furans, mercury and require additional processing and contribute towards increased operational cost and safety risks.
The proposed hydrometallurgical process in comparison accomplishes the recovery of both lithium and cobalt without having to deal with either of the aforementioned problems. It utilizes acidic leaching, which consists of converting entrenched valuable metals into soluble salt ion solutions. These ions are later extracted while impurities remain insoluble to the leaching solution. This innovative design not only boosts cobalt recovery to 98% from 87% via the pyro process, it also boasts 81% recovery of lithium in addition.
HOW WE VALIDATED OUR DESIGN SOLUTION
Extensive theoretical research was performed to incorporate the hydrometallurgical process and its unit operations consisting of reactors into a viable simulation. Kinetics of key process units including the acidic leaching reactor, and chemical precipitation reactors were simulated based on assumptions from established literature sources for this novel process. Due to the unique solvents and feedstock featured in the hydrometallurgical process, a majority of available software was incompatible with our process design. Thus MATLAB was utilized to confirm key calculations including reactor sizing, and validate reaction conversion.
Simulations were also performed on the various heat exchangers present within the plant. Aspen Plus was used to simulate the heat exchangers using the shortcut method of calculation. Exchanger design was chosen to be a shell and tube that operates through countercurrent flow utilizing hot steam entering through the shell. This selection provides optimal heat transfer and integration throughout the plant.
FEASIBILITY OF OUR DESIGN SOLUTION
Profitability Analysis
The total capital investment is $14.2 million. The fixed capital investment is $12.4 million and the calculated working capital is $1.8 million. A large majority of the fixed capital investment came from the equipment and indirect costs such as engineering, supervision, construction, legal expense and contingencies. The annual operating cost was calculated to be $4.7 million. This included material, labor, energy, utilities, waste disposal, maintenance and transportation costs . For revenue, our two products, cobalt and lithium, had a selling price of $76.41 per kg and $35.38 per kg respectively. This led to a yearly revenue after expenses of $684,000 after taxes.
The cumulative cash flow estimation at the end of the 25 years of operation was determined to be $4.3 million. For the net present value, we applied a 2.5% discount rate which gave a value of $330,000. The discounted cash flow rate of return was 3%. The payback period was calculated to be 21 years and the rate of return was found to be 4.85%. These underlying fundamentals tell us that while this project will be profitable, it endures a lot of risk for an average return on investment compared to a relatively safer investment such as bonds which have returned 3% over the last decade.
Sensitivity Analysis
The sensitivity analysis shows that if revenue were to increase by 30%, we would get a rate of return of 15%. Most investors have a benchmark of 10% being a good rate of return so this shows the potential upside if the revenue increases however there is a huge cause for concern if the revenue decreases 10% as our payback period would more than triple to 66 years. This trend is similarly shown for fixed cost. If our company could come to an agreement with the government to subsidize our fixed cost by 30% because of the beneficial environmental impacts, we would have a good rate of return. However, if our estimations are wrong and we go over budget, our rate of return can be cut by half. While both parameters show how our company can be impacted economically, the emphasis is on revenue and how the volatility of lithium or cobalt can dramatically impact our profitability.
DETAILED DESIGN
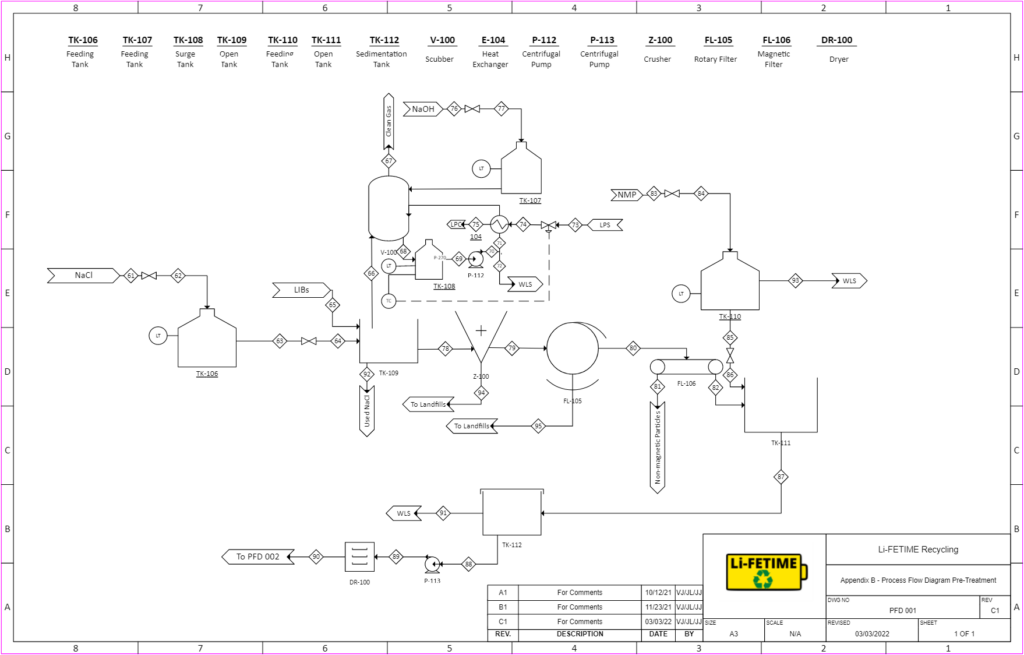
PRE-TREATMENT
The pretreatment process is to reduce the volume of waste lithium-ion batteries and reduce the pollution problem during the recycling process, and on the other hand, it is to achieve the enrichment of valuable metal components.
The pretreatment process mainly accounts for discharging, dismantling and separating, and the separation of the cathode active materials.
Discharging: utilizing sodium chloride solution to immerse the batteries to discard the remaining capacities
Dismantling and Separating: mechanical separation process includes crushing, sieving and magnetic separation, the process is done to get cathode
Separation of Cathode Active Materials: Placing in NMP for 1h at 80℃ and then treat in an ultrasonic bath for 20 min to separate the cathode active material from Al foils
After that, the stream would pass through a sedimentation tank to get LCO sludge. By drying and grinding to get the powder.
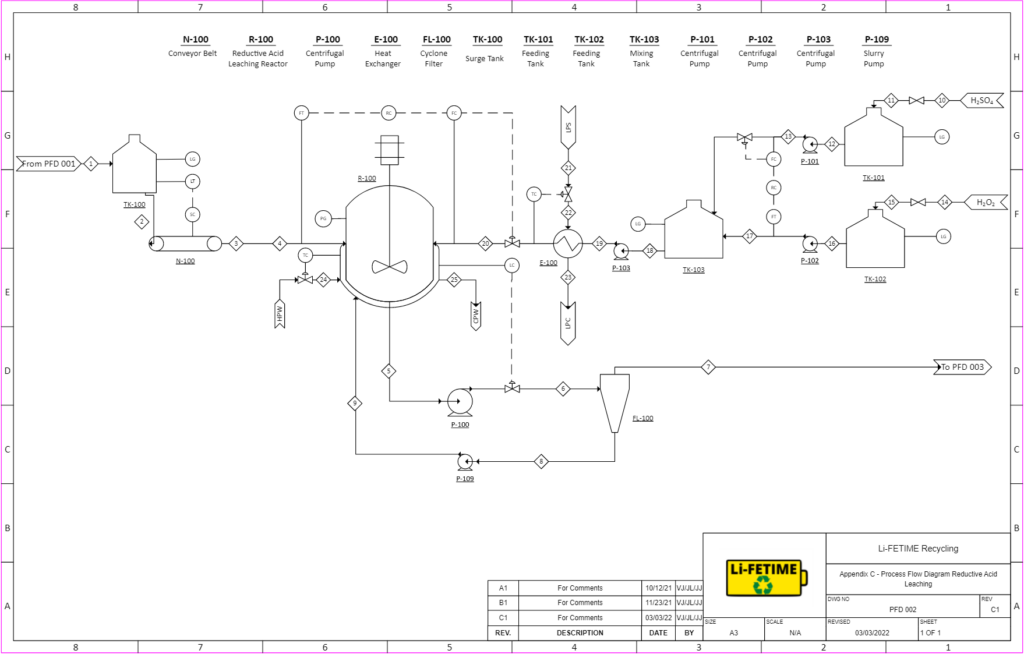
REDUCTIVE ACID LEACHING
Acid leaching is a conventional process used in extractive metallurgy. It will be utilized to dissolve our lithium cobalt oxide (LiCoO2) feed into lithium and cobalt ions, which will later be recovered in the succeeding precipitation reactors.
LiCoO2 from pretreatment will enter the leaching continuously stirred tank reactor (CSTR) as a crystalline solid, where it will be mixed with solvents sulfuric acid and hydrogen peroxide at 80°C.
The reaction in the leaching reactor is:
2LiCoO2 + 3H2SO4 + H2O2 → Li2SO4 + 2CoSO4 + 4H2O + O2
99% of the LiCoO2 will be leached and turned into Li+ & Co2+ions. The unreacted LiCoO2 will be recycled back into the reactor.
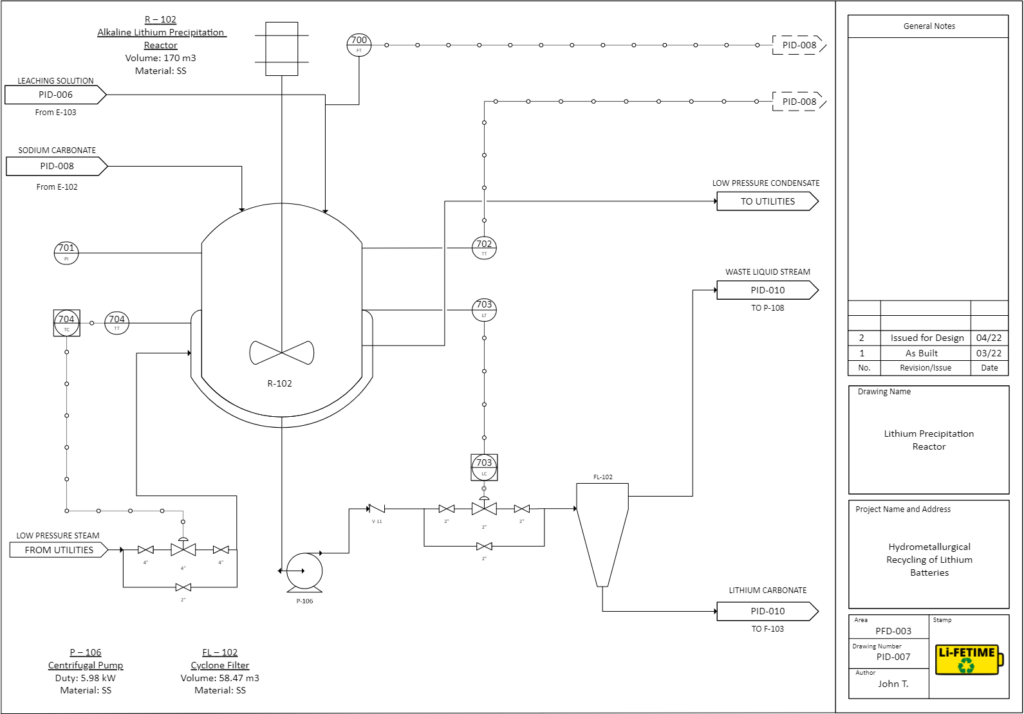
CHEMICAL PRECIPITATION
The leach liquor solution from the leaching reactor will then go through two chemical precipitation steps in two separate CSTR’s.
Cobalt Precipitation: Solution enters the CSTR at 55°C and is mixed with ammonium oxalate ((NH4)2C2O4).
98% of cobalt ions precipitate as cobalt oxalate dihydrate (CoC2O4· 2H2O) – a powder with purity of 97%, that is practically ready for industrial use as a cobalt catalyst and for cobalt metal powder for powder metallurgical applications.
Co2+(aq) + C2O42-(aq) + 2H2O → CoC2O4· 2H2O (S)

Lithium Precipitation: Solution enters the CSTR at 95°C and is mixed with sodium carbonate (Na2CO3).
81% of lithium ions precipitate as lithium carbonate (Li2CO3), with a purity of 99%, which can be directly used for lithium ion battery manufacturing. It is also used as psychiatric medication to treat mania.
2Li+(aq) + CO32-(aq) → Li2CO3(S)

The waste stream will require a thorough method of recycling and disposal as a lot of the solutions present can be recycled back into the plant. However, the waste stream has a significant amount of ammonia that will require a method of safe disposal as ammonia is a pollutant to the environment.
PARTNERS AND MENTORS
Li-FETIME Recycling would like to thank the key individuals who assisted us with our project throughout the Fall 2021 and Winter 2022 semesters.
Firstly, we would like to thank our project supervisor, Dr. Edward (Ted) Roberts, for meeting with us on a weekly basis and providing invaluable insight and guidance over the course of the year.
We would also like to thank Dr. De la Hoz Siegler and Dr. Michael Foley for their vital feedback and suggestions on how to improve our design.
.

SAMPLE DOCUMENTS


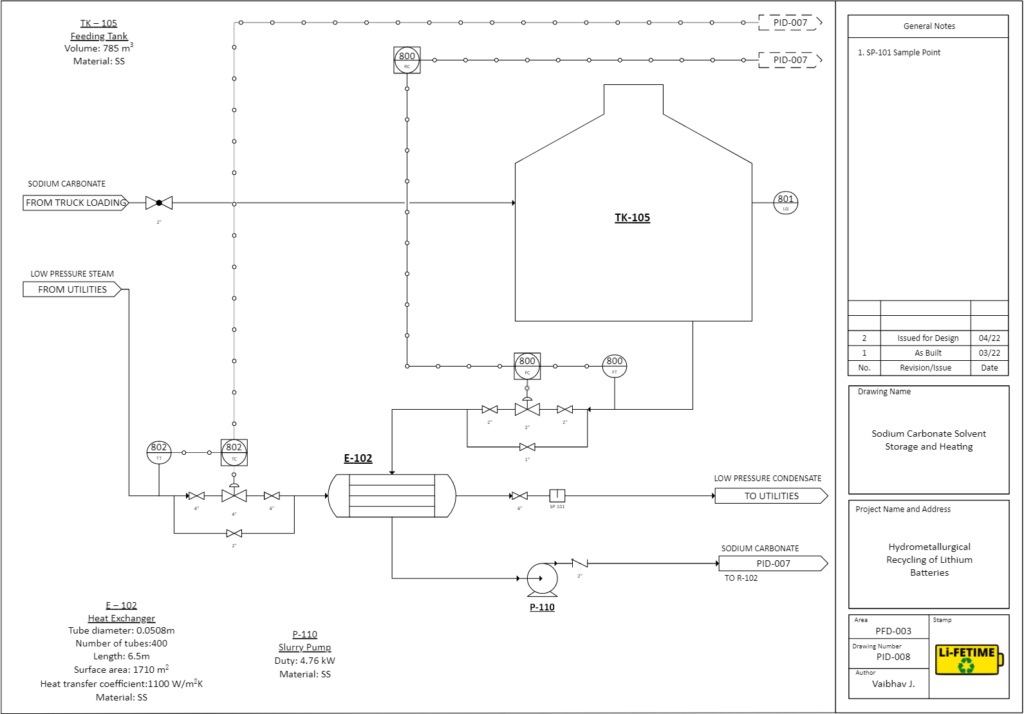
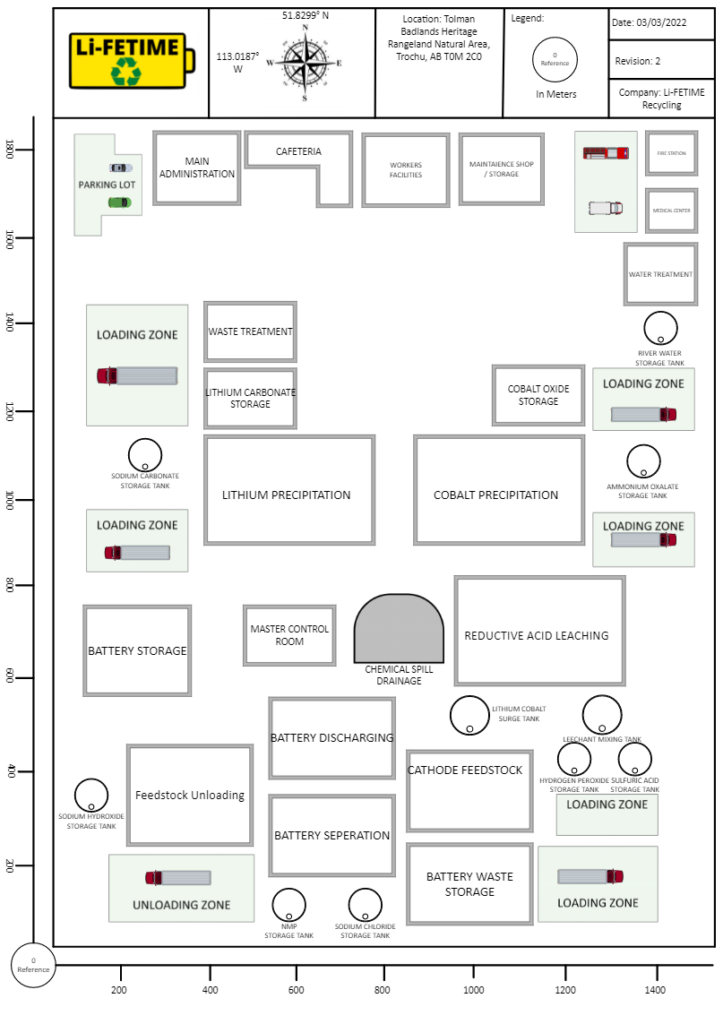
REFERENCES
WORKS CITED
Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6(11), 13611–13627. (accessed Feb 16, 2022).
Sun, L., & Qiu, K. (2011). Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. Journal of Hazardous Materials, 194, 378–384. (accessed Feb 16, 2022).
Peng, C., Hamuyuni, J., Wilson, B. P., & Lundström, M. (2018). Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Management (New York, N.Y.), 76, 582–590.(accessed Feb 18, 2022).
Lithium2021 Data: 2022 forecast: 2017-2020 historical: Price: Quote: Chart. https://tradingeconomics.com/commodity/lithium (accessed Dec 9, 2021).
Cobalt2021 Data: 2022 forecast: 2017-2020 historical: Price: Quote: Chart. https://tradingeconomics.com/commodity/cobalt (accessed Dec 9, 2021)
Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical Recovery of Metal Values from Sulfuric Acid Leaching Liquor of Spent Lithium-Ion Batteries. Waste Manag. 2015, 38, 349–356. (accessed Feb 16, 2022).
Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A Novel Pyrometallurgical Recycling Process for Lithium-Ion Batteries and Its Application to the Recycling of LCO and LFP. Metals 2021, 11, 149. https://doi.org/10.3390/met11010149 (accessed Feb 21, 2022)
Greg McBride, C. F. A. What is a good return on investment? https://www.bankrate.com/investing/good-return-on-investment/ (accessed Mar 2, 2022).
Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6(11), 13611–13627.(accessed Feb 11, 2022).
Zhou, Li-Feng, et al. “The Current Process for the Recycling of Spent Lithium Ion Batteries.” Frontiers in Chemistry, Edited by Moisés Canle et al., https://doi.org/https://www.frontiersin.org/articles/10.3389/fchem.2020.578044/full.
