Project Category: Chemical
Project Overview
More than meets the I2 – A 21st Century Solution to a 100-Year Old Problem
How do you imagine that your life would change if you did not have access to safe and reliable sources of water? In other words, what does water mean to you?
For instance, every person in the developed world has a memory where they found an inexplicable source of enjoyment from water. Whether for you that moment on is a hot summer day, where a cold sip of water instantly brings you refreshment; or just a regular morning where that rich aroma of coffee brings the invigoration to begin your day. The importance of that feeling is where this story begins.
Since the industrial revolution, production of goods, materials and products has increased year after year. The common denominator in majority of these production processes is that they consume, contaminate, or corrupt the integrity of water quality world-wide. Recently, in less developed countries only 50% of health care facilities had basic water services and less than 40% had basic sanitation services. According to WHO/UNICEF, 70-80% of all illnesses in developing counties are linked to the ingestion of contaminated water.
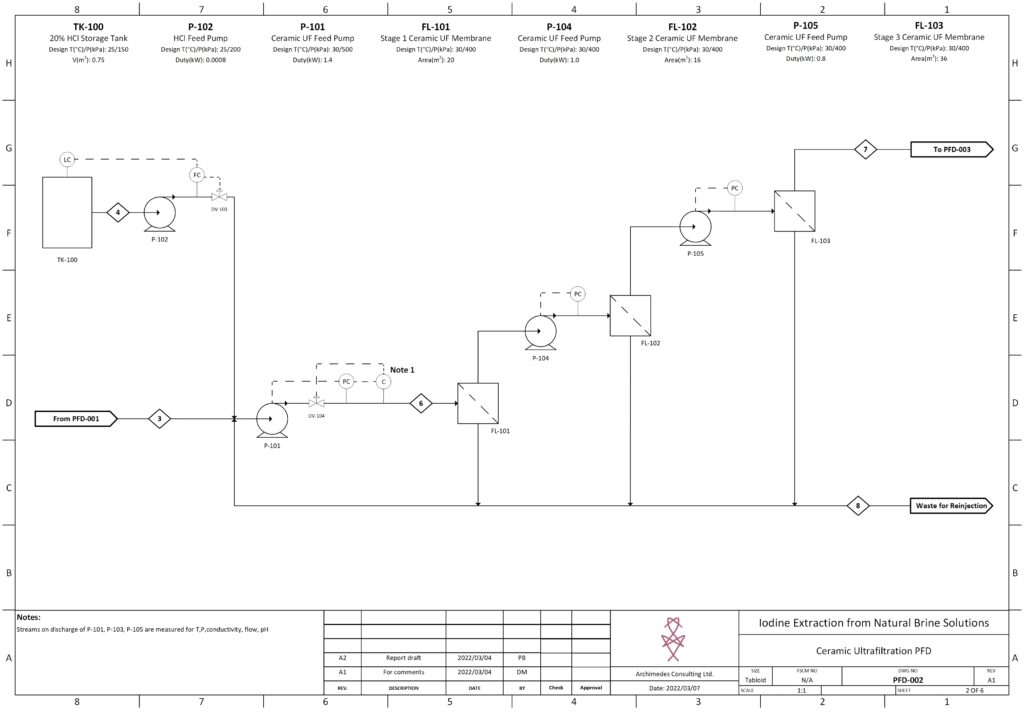
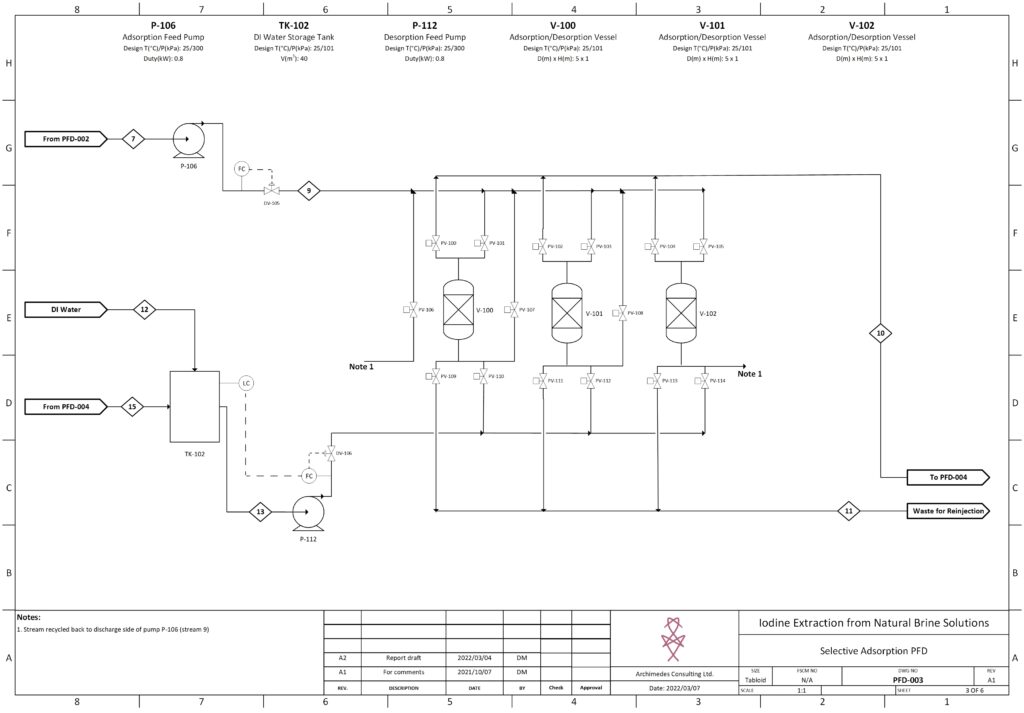
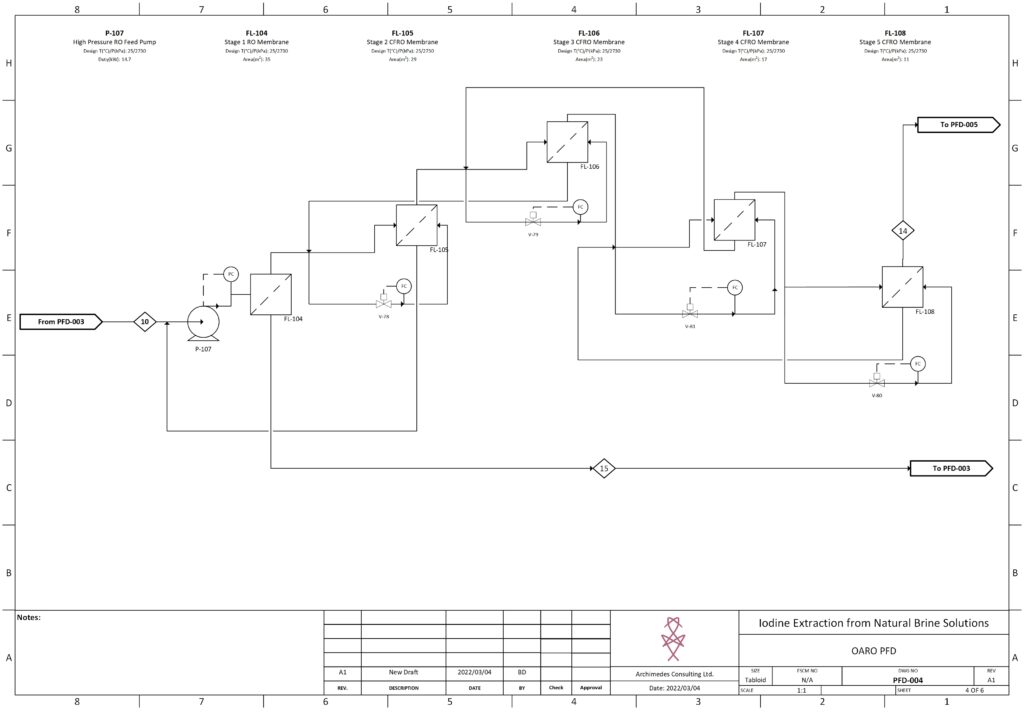
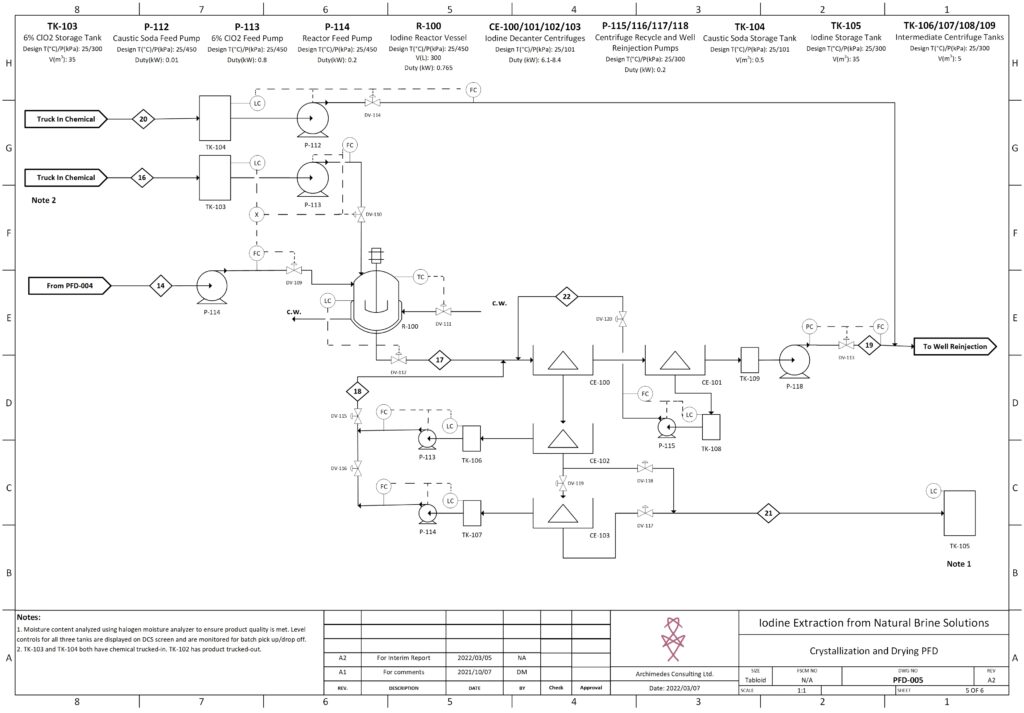
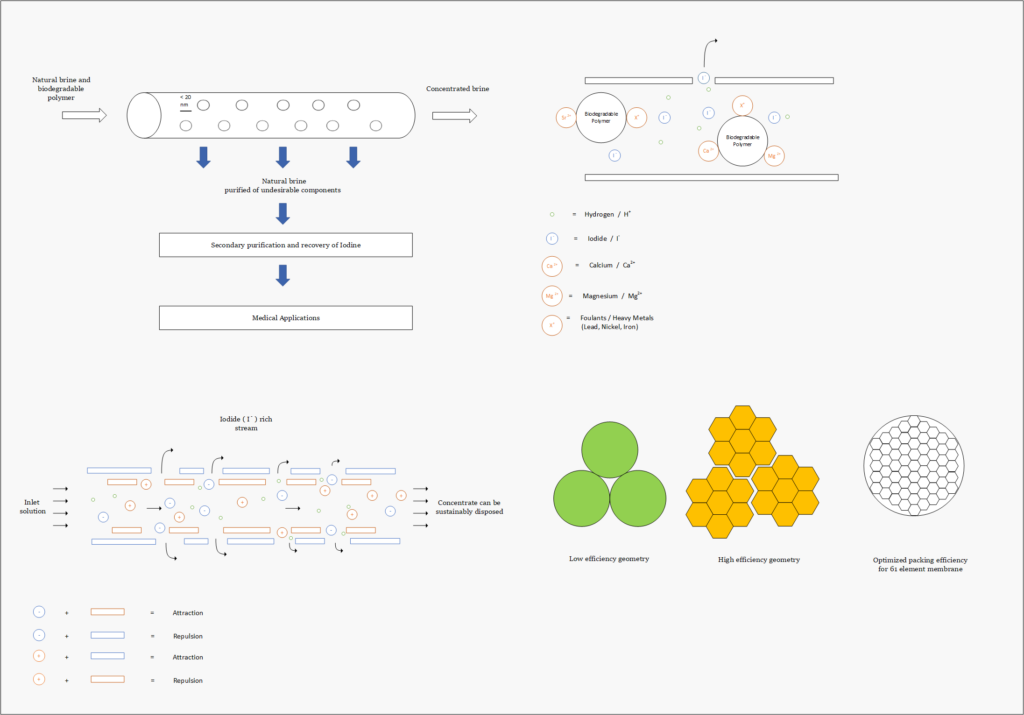
Fortunately, improvements in advanced material science, nanotechnology, and overall processing techniques have allowed for our team to combine existing technologies in a remarkably sustainable novel extraction of iodine. In our process, iodine is extracted from a naturally occurring brine solution using biodegradable compounds that can be safely reinjected after separation. The use of minimal chemical reagents, low energy intensive processing and high efficacy separation techniques promotes the standard that the newly emerging chemical industry has been shifting to hold paramount.
The product is a 99.5% pure iodine crystal flake that holds both high economic and social value. Investment in this process is incredibly attractive due to promising outlooks with respect to financial indicators, utility in cancer treatment, space exploration, and third world water treatment and sanitation. In North America, iodine is used as an antiseptic to ensure highest levels of disinfection in sanitary environments, such as the operating room. The video below is a high level overview of the designed process.
Meet our team
Patrick Buck
Patrick Buck is in his final year of Chemical Engineering at the University of Calgary. Over the last couple years, Patrick Buck has been developing techniques and skills in artificial intelligence; Furthermore, he is professionally certificated in Machine Learning by IBM and has received credentials from Stanford University. His ambition is to improve the efficiency of business operations and physical processes, which has been the focus of his most recent work. Patrick currently works for TD Bank, where he is expanding his understanding of risk management. In the capstone design, his primary focus has been on the preconditioning and nanofiltration membrane units.
Drew Moxon
Drew Moxon is a fifth-year student at the University of Calgary in a unique dual-degree program studying Chemical Engineering and Finance. During his undergraduate studies, Drew participated in many entrepreneurial activities including placement as a finalist in the RBC Fast Pitch Competition for a high-growth startup. Drew is passionate about green technology and innovation in chemical process design and is currently developing his skills at Summit Nanotech Corporation. For the capstone design project, Drew focused mainly on selective adsorption operation and optimization.
Bolin Du
Bolin is a fifth-year student at the University of Calgary pursuing a dual degree in Chemical Engineering and Finance. In University, he has held leadership positions in the Combined Engineering and Business Association and Chemical Engineering Student Society. Since 2018 Bolin has worked at Suncor and Gibson Energy in various roles including process engineering, supply chain, project engineering, drilling engineering, reservoir engineering, treasury, and corporate finance. For the capstone design project, Bolin’s focus was on the concentration unit and financial modeling.
Ian West
Ian West is in his final year of Chemical Engineering at the University of Calgary, where he has involved himself in various organizations including the Petroleum & Engineering Society and the Engineering Leadership Program. During his degree, Ian has helped tutor high school students in STEM subjects by working part-time work at Alberta Diploma Prep, and more recently, Paper. The last two summers Ian was contracted by Global Raymac where he completed field work on commercial energy projects that included Trans Canada’s 2021 NGTL System Expansion. For the capstone design project, Ian’s primary focus was design and optimization of the chemical precipitation unit.
Nolan Akins
Nolan Akins is a chemical engineering and finance student with co-op experience in conventional oil operations, oil sands process innovation, and merchant banking. He is passionate about the world-class Canadian energy industry and will be entering the field full-time post-graduation with Imperial Oil. During his undergraduate studies, he was involved with many organizations in leadership and coaching roles like Special Olympics Basketball, Students for Special Olympics, Combined Engineering Business Association, and North Central Basketball Club. For this capstone design project, Nolan focused mainly on the drying unit, financial modelling, and economics.
Details about our design
Historical Significance of Process
Historically, iodine has been recovered on a commercial scale from seaweed. In this classical approach the seaweed is dried and burned, ash is leached using water and the remainder is dosed with sulfuric acid before being vaporized and sublimed to isolate the desired element.
Production from brines began in 1854 and to this day remains prevalent. Due to low concentrations of the desired element, large quantities of brine typically must be processed to operate economically. Highly unfavorable process conditions have led to the use of inorganic chemical substances to treat the brine. These inorganic agents include aluminum sulfate, ferric chloride, and poly-aluminum chloride. Inorganic chemical treatment is highly effective in treating brine; however, substantial volumes of sludge that are generated can lead to serious health problems for consumers.
What Makes Our Design Innovative?
“Standing on the shoulders of giants”
– Isaac Newton (1675)
Conventional methods use energy intensive techniques with energy demands an order of magnitude greater than modern techniques. These methods including thermally driven and chemical driven desalination processes treat the brine using strategies that are detrimental to the environment. Evaporative techniques require significant amounts of energy and chemical methods produce considerable amounts of harmful waste through the addition of dangerous chemicals.
The proposed design intends to present a robust set of technologies that can be utilized in an array of applications that extends beyond iodine recovery. The use of highly selective proprietary materials allows for potential investors to tailor solutions to their specific application. Tuning the behavior of the process has been implemented into our design to accommodate a diverse range of applications. These tunable selections include the active layer properties of the nanofiltration membranes, selective properties of the sorbent material and oxidation agent chosen for the process.
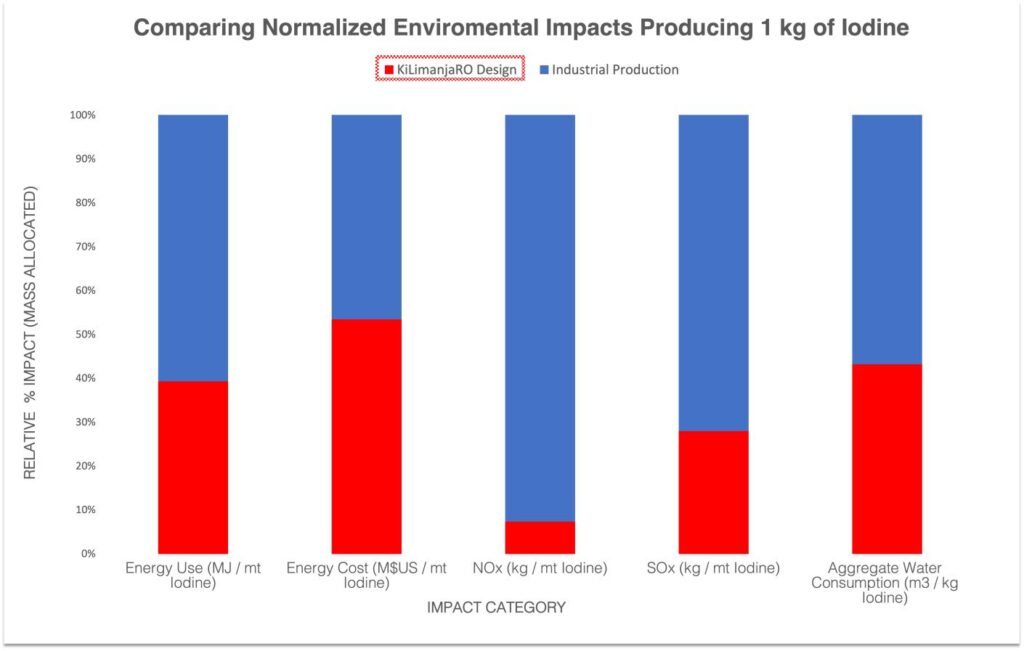
Our design does not reinvent the wheel but instead applies the existing body of knowledge found in academic literature and couples them with the latest technologies. Supported by previous pilot operations we aim to achieve the highest degree of productivity, and effectiveness in our practical solution. We believe that through the use advanced materials and the proposed techniques we can achieve extraordinary results concerning environmental appeal. These benefits are comparable to existing processes with respect to the energy demands, greenhouse gas emissions and quantities of produced chemical waste.
How Our Solution Addresses Practical Issues
Global iodine demand is rapidly growing; from USD$ 853M in 2020 to a projected USD$ 1.1B in 2027. Current processing technologies are unsuitable for expansion as they are environmentally damaging and require immense raw materials and infrastructure for their operation. The chemical mining and processing industry needs an innovative solution to extract iodine. Our solution is a low-energy, low-chemical, iodine extraction process that produces a high purity iodine product.
How We Validated Our Design Solution
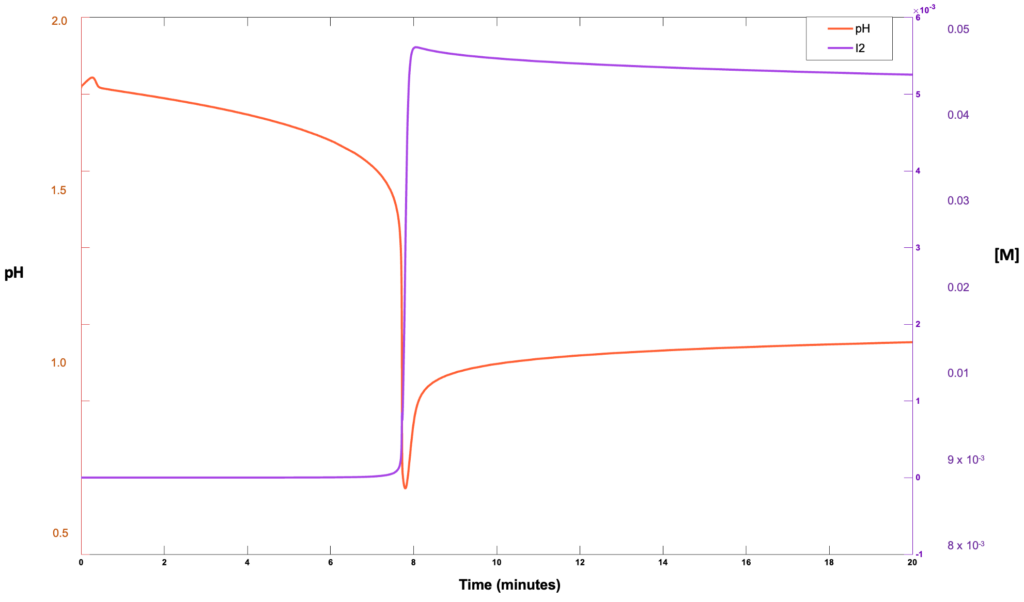
Heat and material balances were performed for each unit operation and compared across literature and pilot operation results for similar conditions. The selection of the relevant equipment and sizing was completed in accordance with multiple engineering design textbooks, which provide standard practice and operation specification selection guidelines for each individual unit process. Comparison using multiple methods of validation ensured that the trajectory of the solution was constrained to within tolerable thresholds to permit economical operation. Process simulations using python and matlab were used to solve systems of differential equations relevant to the underlying process.
Machine learning and deep neural networks were used in what is known as a physics informed neural network. Using this method the solution vector space of physically realizable conditions can be quantified and deviation from plausable states can be evaluated with a loss function. For visual intuition below is the solution to the laplace equation in cylindrical coordinates.
Closed form solutions of various equations were evaluated, which would allow for future data collection endeavors to tailor predictive modelling to conditions imposed by the underlying physics. Attached below is a closed form solution to the modified bessel functions of the first kind.
Feasibility of Our Design Solution
On a technical basis, our design solution is based on various considerations identified from academic papers with appropriate fundamental equations, we created models for each unit to accurately simulate our process outputs and operating conditions. The specific operating conditions enabled us to source equipment and utilities to create a financial model that justify the financial profitability. Finally, through an optimization process, we were able to reach a base case NPV of $ 2.9M.
Partners and mentors
We would like to personally acknowledge and thank Dr. Kunal Karan for his time, effort, and dedication to this project. Dr. Karan has devoted countless hours to educate, collaborate with, and inspire our group. Not only do we believe Dr. Karan’s contributions have significantly enhanced this project, but also our approach to solving other real-world engineering problems. Thank you, Dr. Karan.
Our photo gallery

References
World Health Organization. https://who.int/news-room/fact-sheets/detail/drinking-water
Okaiyeto, K.; Nwodo, U.U.; Okoli, S.A.; Mabinya, L.V.; Okoh, A.L.; Implications for public health demands alternatives to inorganic and synthetic flocculants: bioflocculants as important candidates. MicrobiologyOpen [Online] 2016, 5(2), 177-211, https://doi.org/10.1002/mbo3.334.
Voutchkov, N. Desalination Engineering Planning and Design, 6th ed, McGraw Hill: New York, 2013; pp. pp.8 – 673.
Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. J Nutr. [Online] 2008, 138(11), 2060–2063. https://doi.org/10.1093/jn/138.11.2060 (accessed Oct. 12, 2021).
R. Singh, Membrane Technology and Engineering for Water Purification, 2nd Edition, Elsevier: Colorado Springs, CO, 2015; pp. 1 – 442.
Mordor Intelligence LLP.; Iodine Market- Growth, Trends, COVID 19 Impact, and Forecasts (2021 – 2026); https://www.mordorintelligence.com/industry-reports/iodine-market (accessed Oct. 12, 2021), March, 2021, ID: 6039522
Jansen, M. Sublimation of iodine: Rise and fall of a misconception. Crescent School. https://uwaterloo.ca/chem13-news-magazine/october-2015/feature/sublimation-iodine-rise-and-fall-misconception (accessed February 7, 2022).
Green, D., Southard, M. Perry’s Chemical Engineers’ Handbook. https://www-accessengineeringlibrary-com.ezproxy.lib.ucalgary.ca/content/book/9780071834087/toc-chapter/chapter9/section/section14. Published 2019 (accessed Dec 1, 2021).
Hutchins, M.; Robinson, S.; Dornfeld, D. Green Manufacturing and sustainable … – escholarship.org. https://escholarship.org/content/qt84z0z75t/qt84z0z75t.pdf?t=n8tbjc (accessed Mar 3, 2022).
Gastaldi, R.; Muraca, M.; Beltramo, A.; Poggi, E.; Iodine deficiency and its consequences for cognitive and psychomotor development of children, Italian Journal of Pediatrics [Online] 2014, 40, https://doi.org/10.1186/1824-7288-40-S1-A15 (accessed Oct. 12, 2021).
Zimmermann, M.B.; Research on Iodine Deficiency and Goiter in the 19th and Early 20th Centuries. J Nutr. [Online] 2008, 138(11), 2060–2063. https://doi.org/10.1093/jn/138.11.2060 (accessed Oct. 12, 2021).
