Project Category: Chemical
Our goal is to develop a semi-continuous platform for the manufacturing of antibodies.
Team Adaptygen

Project Overview
What are antibodies?
The human immune system is the innate defense mechanism against various pathogens that may cause a wide variety of diseases. It is comprised of two main responses: 1) humoral (antibody) and 2) cellular (cell-mediated). The humoral immune system can produce specific antibodies against foreign invading antigens, allowing for site-specific resistance to potential disease-causing pathogens.
Monoclonal antibodies (mAbs) are a subset of antibodies that specifically bind to one (mono) epitope of an antigen, which offer a promising method for high selectivity and precision medicine.
Industrial manufacturing processes operate primarily as batch, although continuous systems are desired to produce large, consistent, and reproducible amounts of monoclonal antibodies at moderate costs.
Adaptygen has designed, modeled, and proposed both an upstream and downstream continuous manufacturing process capable of producing 100kg of antibodies annually.
Applications of Monoclonal Antibodies
Therapeutics
Promising potential therapeutic applications in the treatment of cancer, autoimmune diseases, transplant rejection, cardiovascular disease, viral infections, and many other diseases.
Diagnostics
Detection of various conditions including pregnancy, HIV, bacterial infections, influenza, and tumor-specific cancers.
Clinical
Purification of drugs, aids in live imaging of specific tissue, delivery of chemotherapy, flagging cancer cells, and development of CAR-T cells.
Meet team Adapytgen

Alexis Pawluk
Chemical w/ Biomedical Specialization

Emilie Gysel
Chemical w/ Biomedical Specialization

Joshua Feddema
Chemical w/ Biomedical Specialization

Kristian Corpuz
Chemical w/ Biomedical Specialization

Sanchit Chopra
Chemical w/ Biomedical Specialization
Design Details
Gap in Industry

At Adaptygen, we are designing a first-in-kind Canadian manufacturing facility operated as a contract-based manufacturer to produce 100 kg mAbs annually.
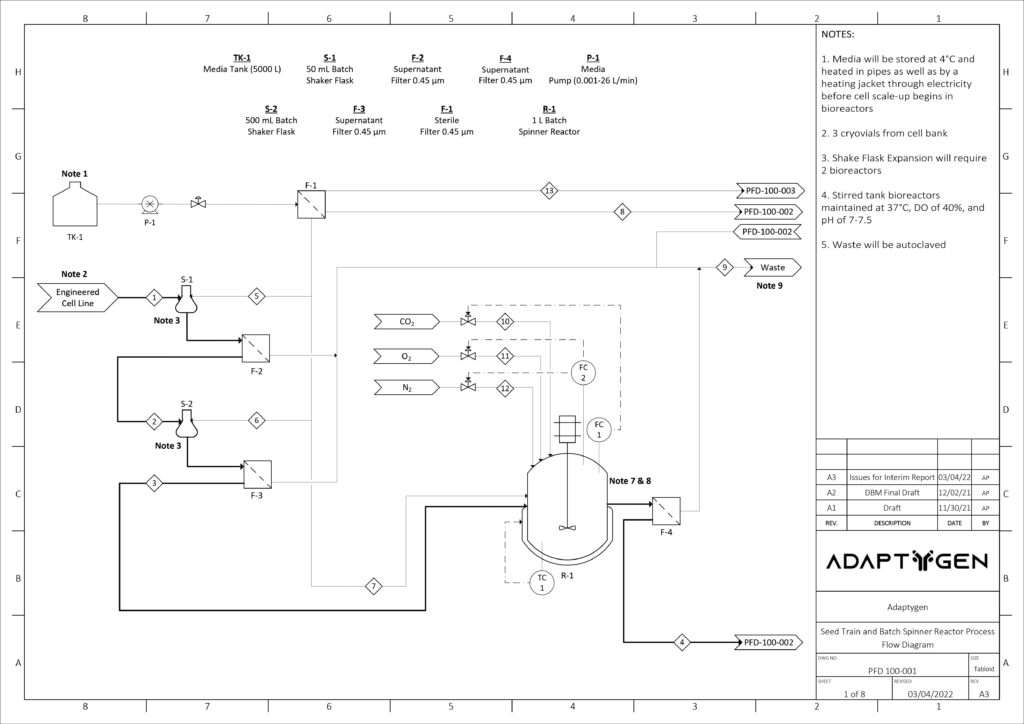
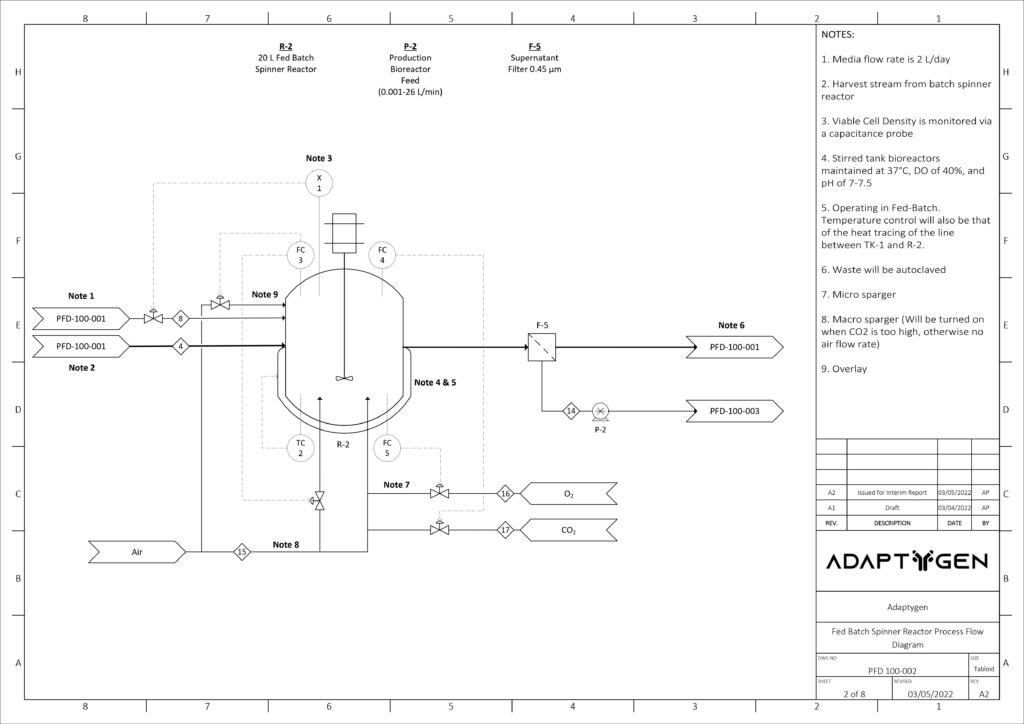
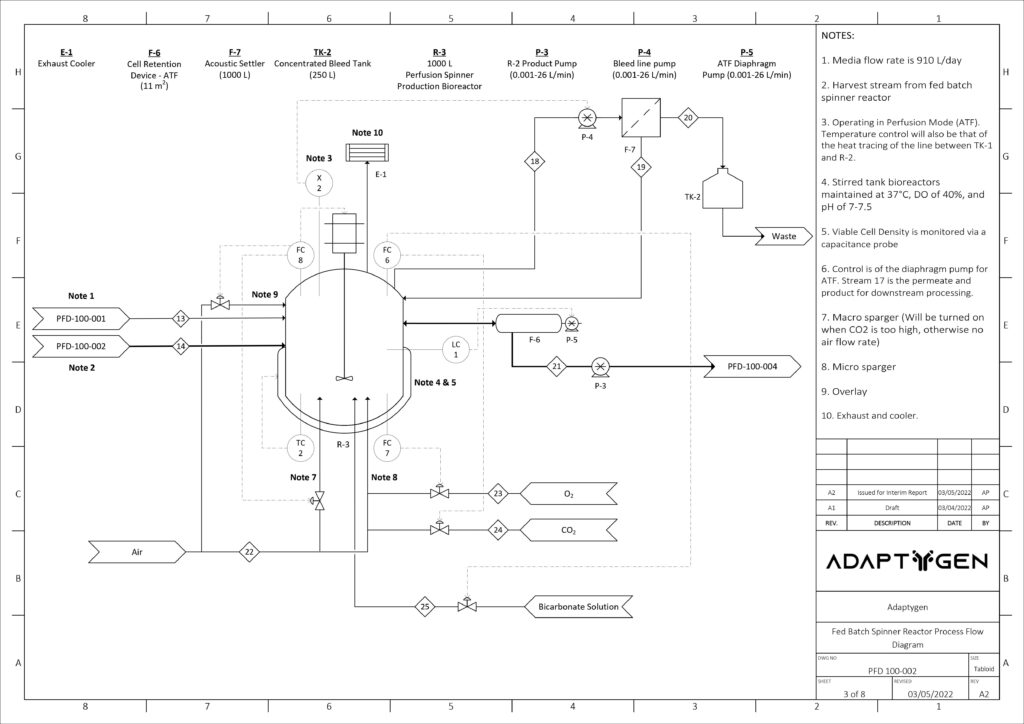
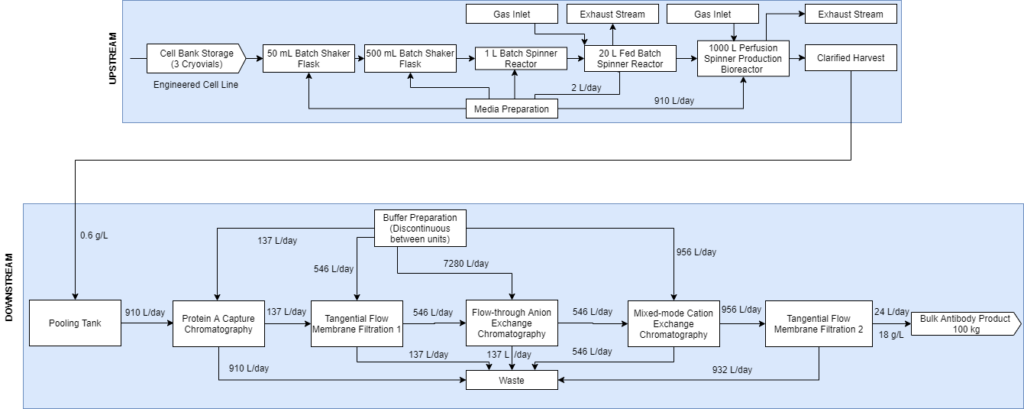
Upstream Cell Expansion Process
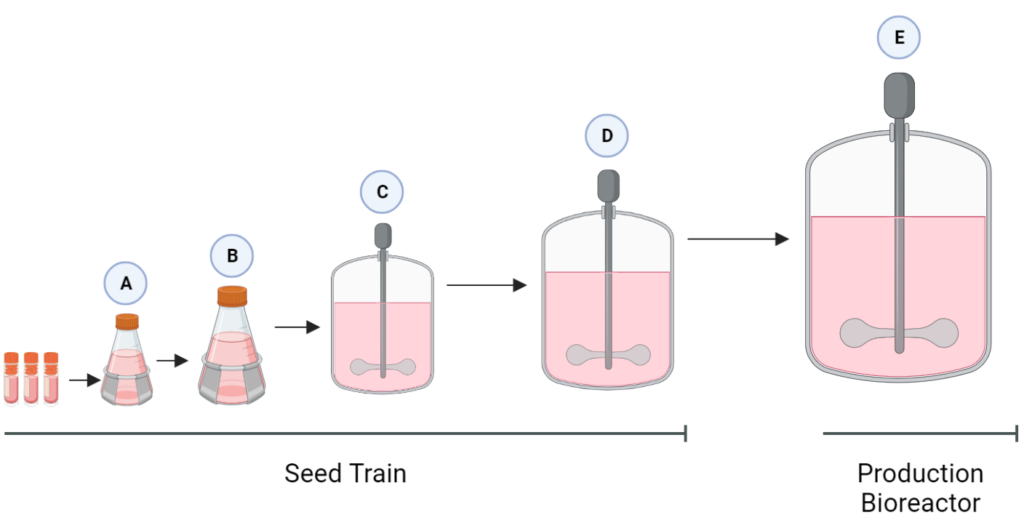
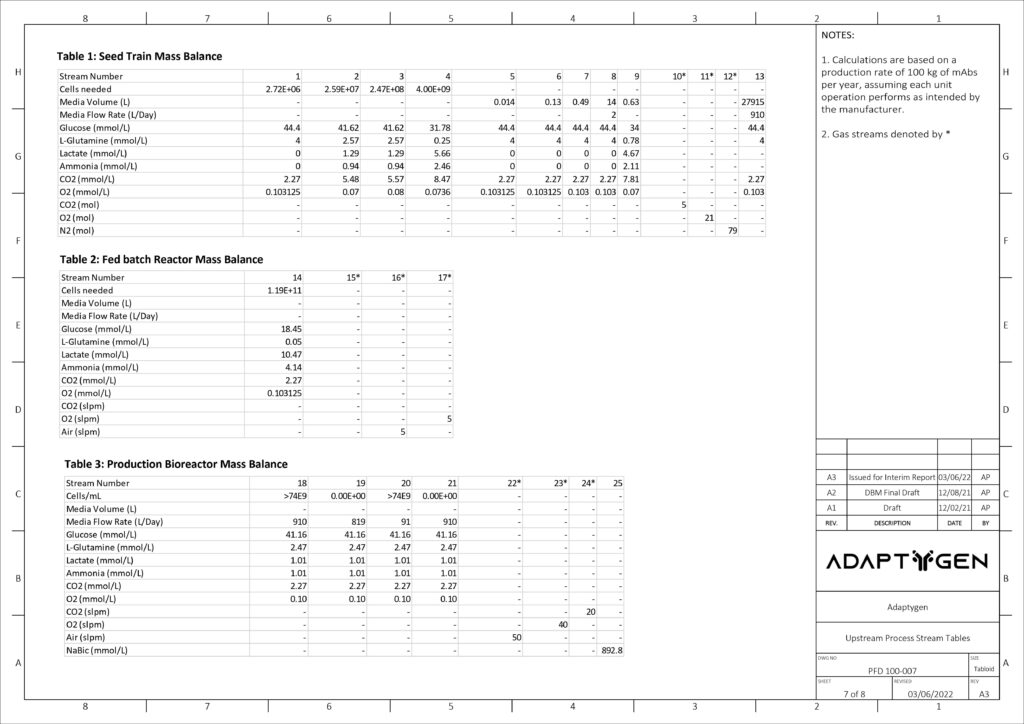
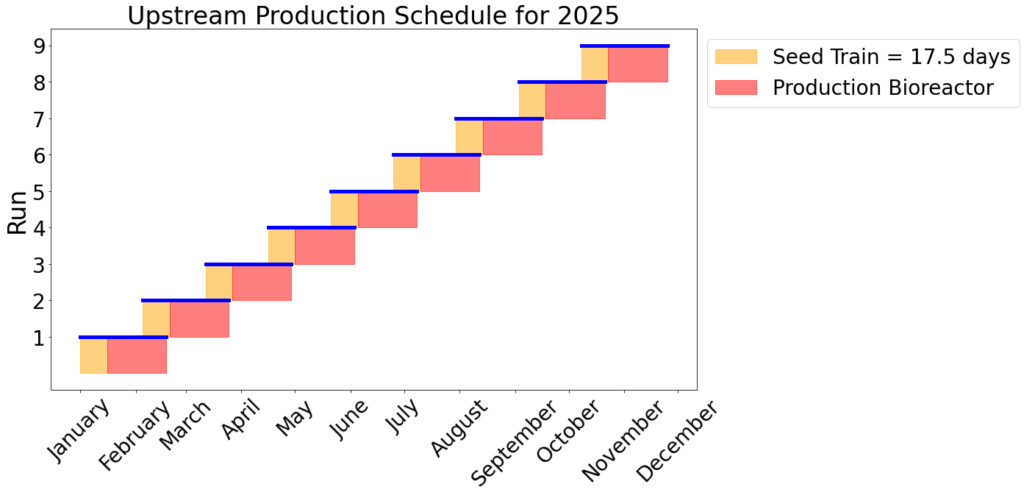
The purpose of the seed train is to generate an adequate number of cells for the inoculation of the production bioreactor. Adaptygen’s seed train consists of Corning shake flasks, sized 50 (A) and 500 mL (B), 1 L batch spinner bioreactor (C), and a 20 L fed-batch spinner bioreactor (D). The production bioreactor (E) consists of a 1000 L perfusion spinner reactor. The production bioreactor can support high viable cell density (VCD) due to the continuous media exchange rate defined by the cell-specific perfusion rate, the cell bleed to control VCD, and the use of micro and macro spargers to ensure efficient gas exchange occurs within the reactor.
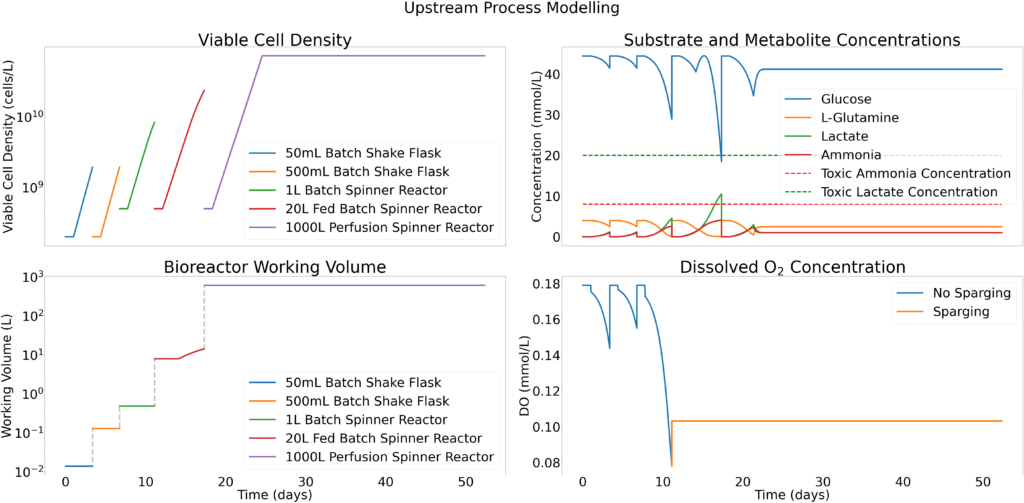
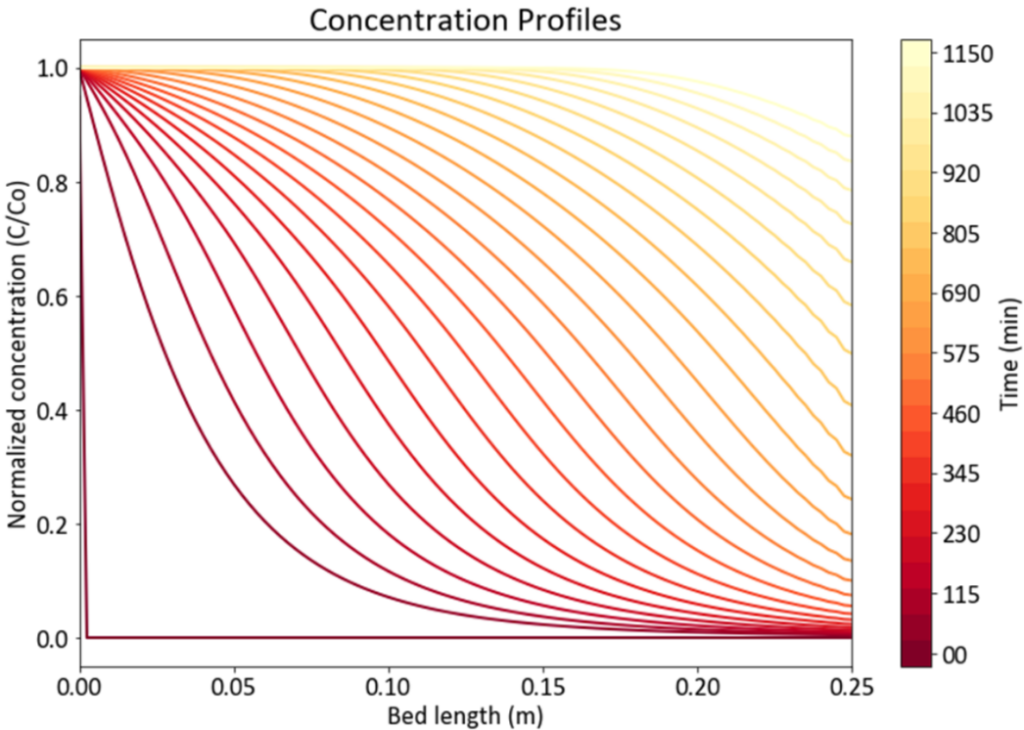
Kinetic models have been utilized to determine the reactor sizes and seed train timeline. The image on the right shows the modeling results of CHO cells for the seed train. Nine production bioreactor runs at a length of 35 days will occur annually in order to reach the targeted mAb yield of 100 kg/year.
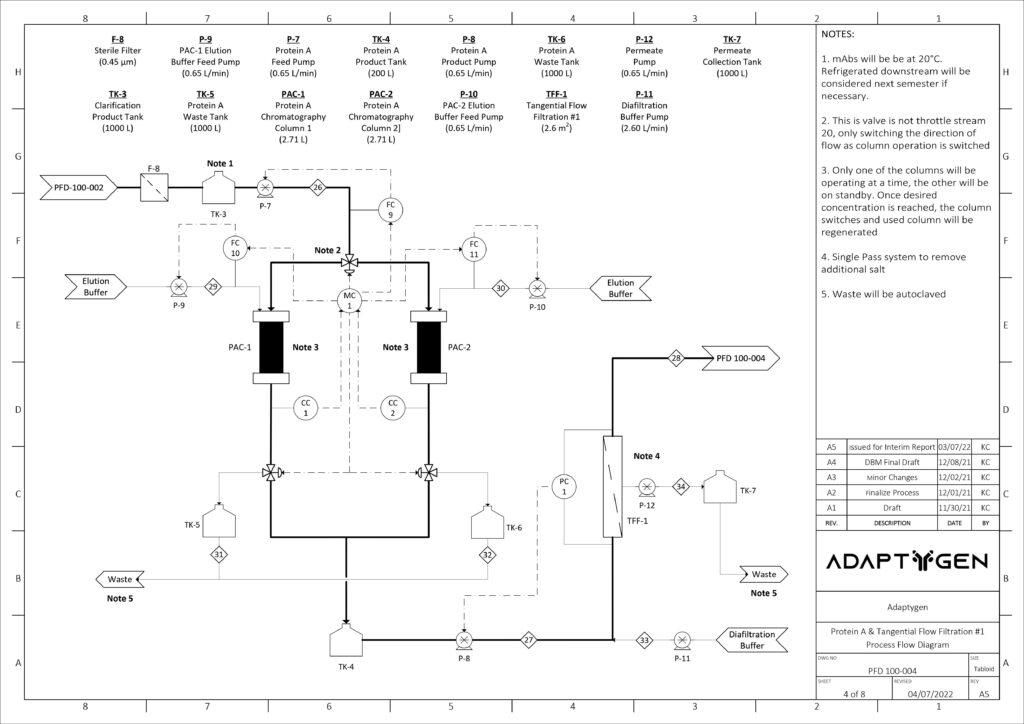
Downstream Purification Process
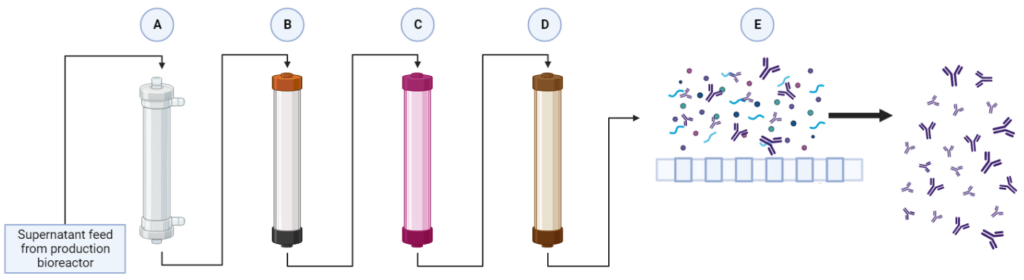
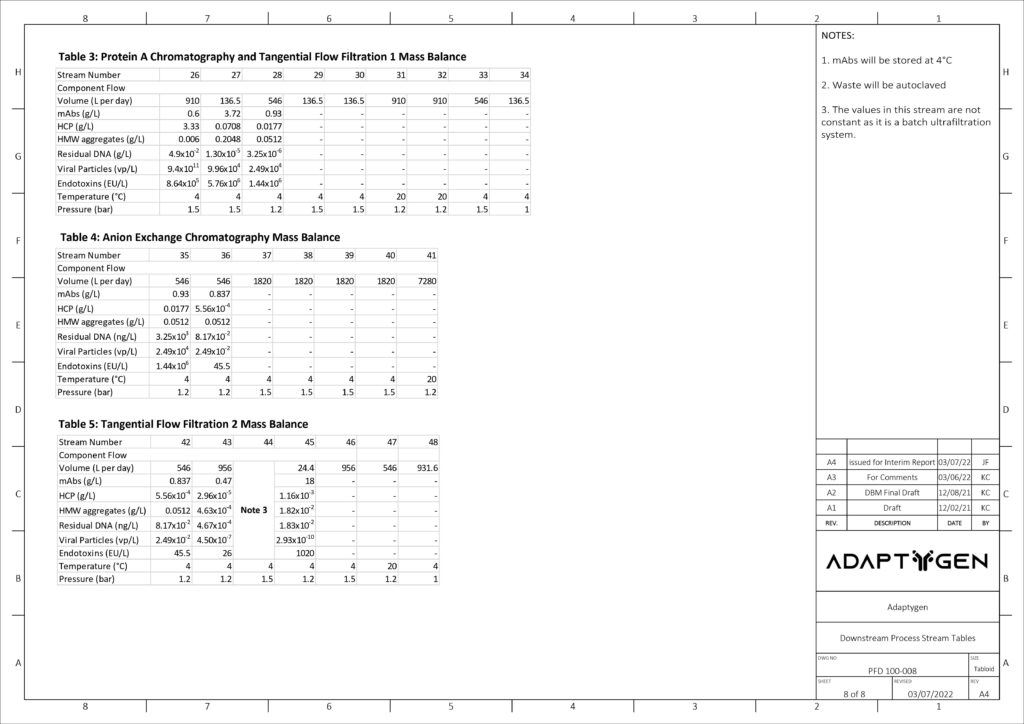
(A) Alternating Tangential Flow Separation: Adaptygen’s perfusion system utilizes alternating tangential flow separation (ATF) as a cell retention device for continuous media exchange that allows for the maintenance of the peak viable cell density.
Diafiltration – Tangential Flow Filtration #1: Adaptygen’s first Tangential Flow Filtration (TFF) unit will use a hollow fibre module in a constant volume diafiltration system to primarily desalt the supernatant that is processed from protein A chromatography.
Hollow fibre modules were chosen over cassettes as they offer high packing density, high surface-to-volume ratio, and a smoother and gentler separation. The unit will be 0.415 m long with a surface area of 2.6 m2.
(B) Protein A Capture Chromatography: Protein A chromatography capture of mAbs is the industry standard for primary separation. MabSelect SuRe LX resin was chosen due to its high dynamic binding capacity and superior clearance of HCP. To fully account for the kinetics, a general rate model (GRM) was developed. The GRM works by performing a differential mass balance in the void space and at radial pore positions within a resin bead for each axial position in the column.
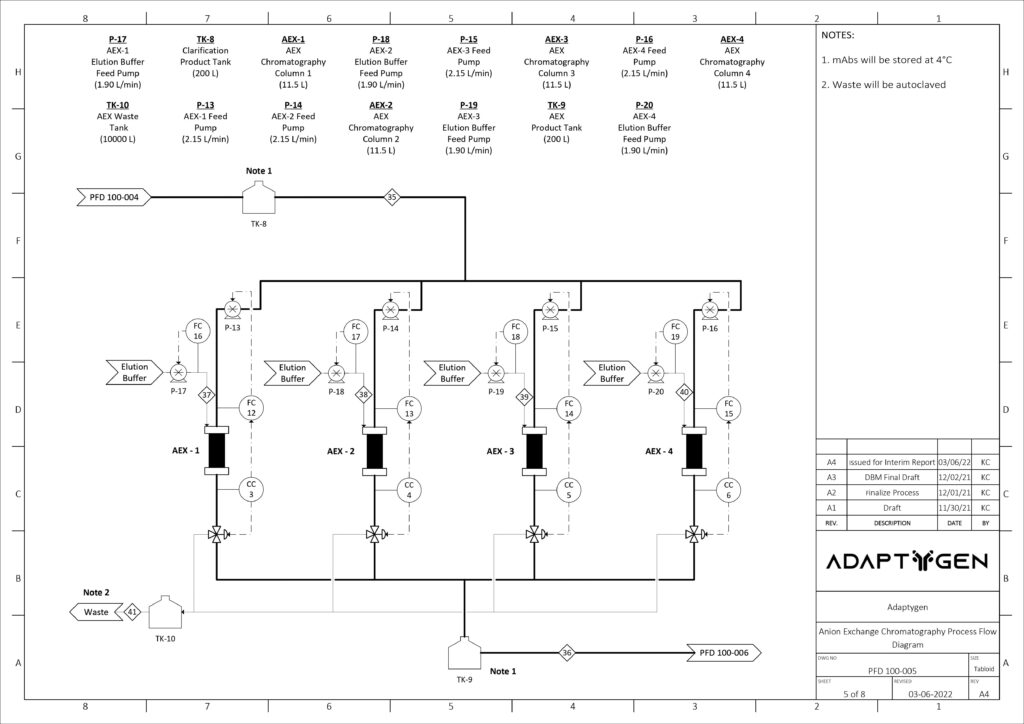
(C) Anion Exchange Chromatography: AEX exploits net surface charges to separate impurities from the mobile phase. The AEX column will operate in flow through mode, where impurities bind to the resin while the mAbs pass through the column. The chosen resin for the operation is Nuiva Q due to its high-performance polishing and data availability. The GRM developed for Protein A Chromatography was used again to provide comprehensive modelling of the AEX column.
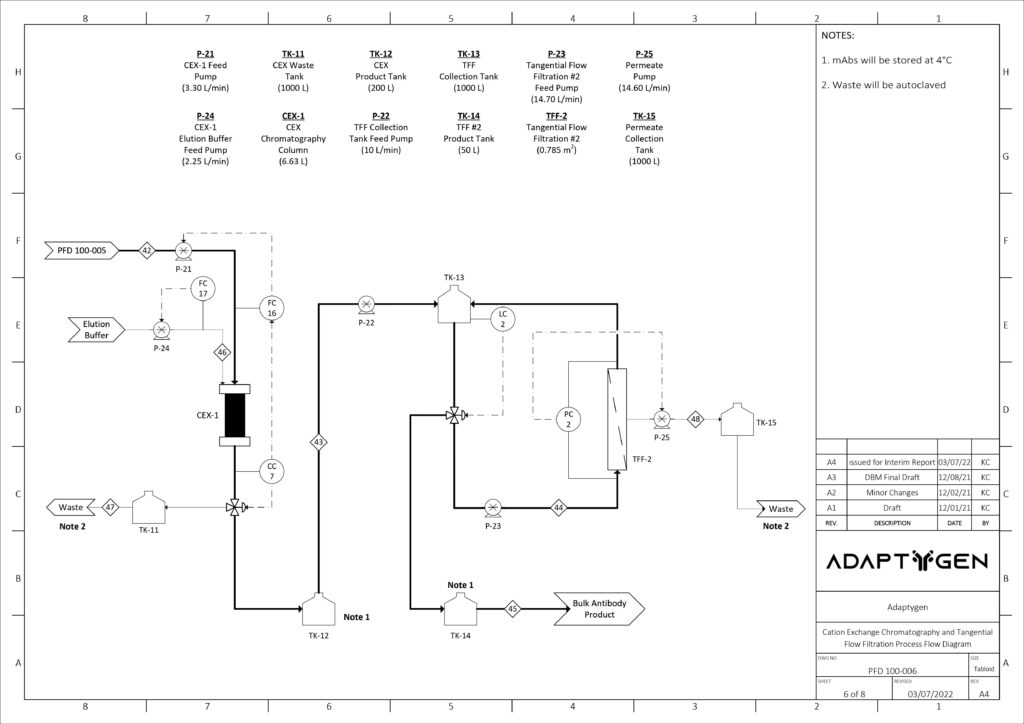
(D) Multi Modal Cation Exchange Chromatography: MMCEX is the last chromatography column in the downstream operation and completes the final polishing. Nuvia cPrime was selected over other options because it has a sharper breakthrough curve with marginally higher dynamic binding capacities.
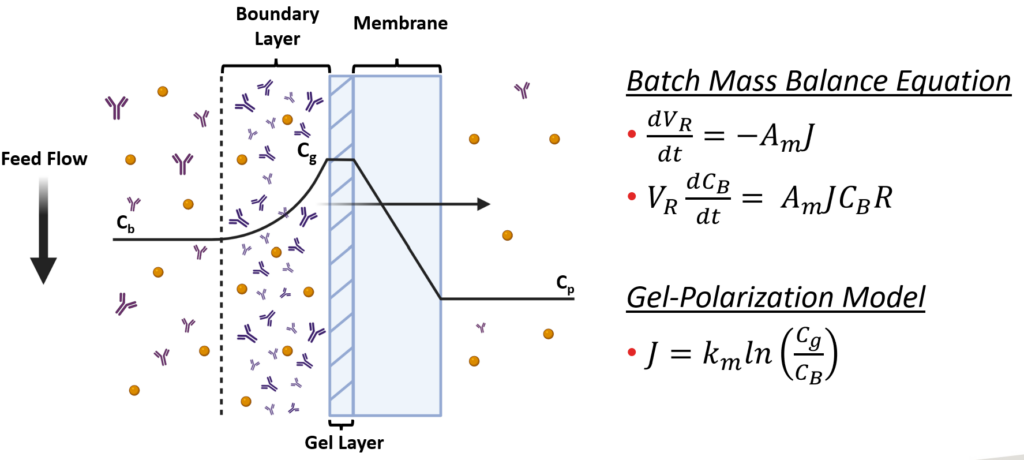
(E) Ultrafiltration – Tangential Flow Filtration: The final unit of the downstream process is ultrafiltration through a TFF system. This concentration step involves the supernatant flowing tangentially across a membrane, allowing impurities to flow across the membrane into a permeate stream while mAbs remain in the retentate stream. The model was based on Gel Polarization Model where the bulk concentration of the supernatant is related to the permeate flux and wall or gel concentration
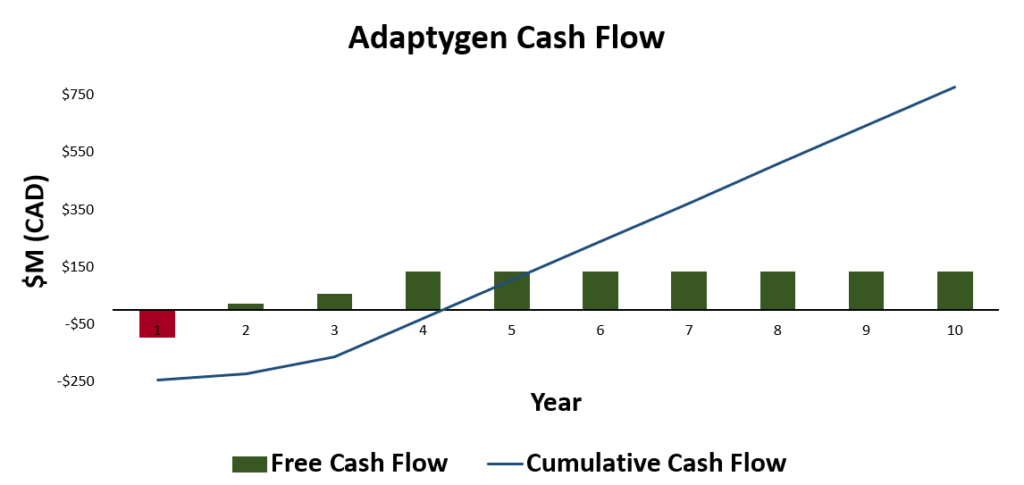
Feasibility and Economic Analysis
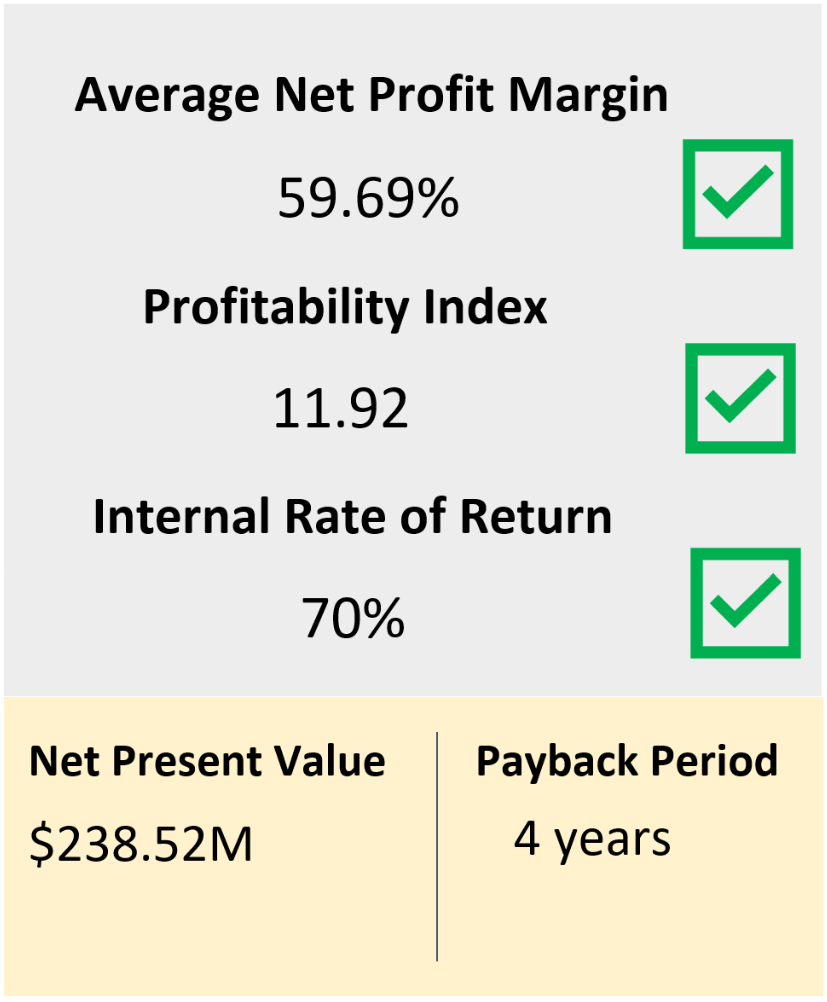
Sensitivity Analysis
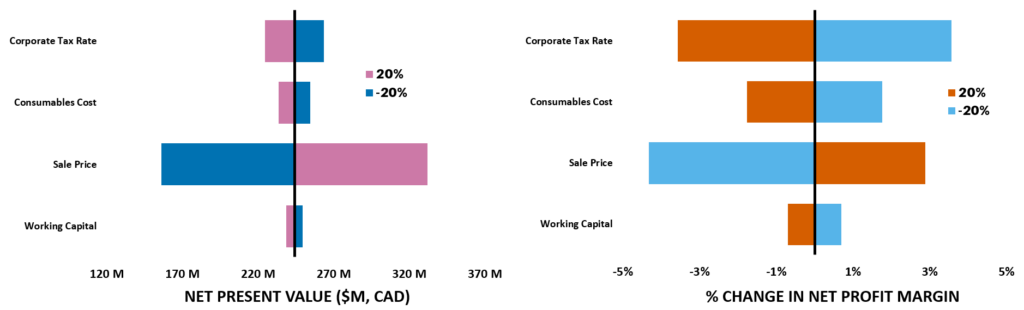
This analysis shows that the sale price has the largest effect on both NPV and net profit margin. Additionally, the corporate tax rate has a significant impact on net profit margins. The consideration of the impact of corporate tax rate can drive executive decisions on where to expand the plants operation in the future (outside of Canada). As the consumable cost did not have a large impact on NPV or net profit margins, further considerations into improved CHO cell media composition and growth additives can be considered to improve productivity. Additionally, as the fixed assets (land cost and equipment purchase, not shown) is the largest percentage of the total source of working capital, further volume expansion (both larger equipment and larger overall process volume) can be considered in the future for expanding the business.
Partners and Mentors
Adaptygen team members would like to thank the following individuals for their support and assistance throughout the Fall 2021 semester.
Firstly, we would like to thank our supervisor, Dr. Michael Kallos, for taking the time and meeting with us weekly, providing us insightful considerations regarding our design and always motivating us during a busy semester.
We would also like to thank our industrial supervisor, Dr. Jeffrey Cohen, from Johnson and Johnson for mentoring us regarding industrial processes and guiding us in achieving our project goals. Thank you for being available to meet with us amidst your busy schedule; your knowledge regarding the project has been innumerable.
Additionally, we would like to thank Casey Mihal from Sartorius and Krishna Panchalingam from Lonza for providing us with technical support regarding our process. We are grateful for their support and mentorship which has allowed us to understand our specific unit operations in more detail.



Our Photo Gallery
References
A. Mahmuda et al., “Monoclonal antibodies: A review of therapeutic applications and future prospects,” Tropical Journal of Pharmaceutical Research, vol. 16, no. 3, pp. 713–722, Apr. 2017, doi: 10.4314/tjpr.v16i3.
R. M. Lu et al., “Development of therapeutic antibodies for the treatment of diseases,” Journal of Biomedical Science 2020 27:1, vol. 27, no. 1, pp. 1–30, Jan. 2020, doi: 10.1186/S12929-019-0592-Z.
H. Kaplon and J. M. Reichert, “Antibodies to watch in 2021,” https://doi.org/10.1080/19420862.2020.1860476, vol. 13, no. 1, 2021, doi: 10.1080/19420862.2020.1860476.
K. H. Liu, “The history of monoclonal antibody development – Progress, remaining challenges and future innovations,” Annals of Medicine and Surgery, vol. 3, no. 4, pp. 113–116, Dec. 2014, doi: 10.1016/J.AMSU.2014.09.001.
G. J. Weiner, “Building better monoclonal antibody-based therapeutics,” Nature Reviews Cancer 2015 15:6, vol. 15, no. 6, pp. 361–370, May 2015, doi: 10.1038/nrc3930.
A. A. Shukla and J. Thömmes, “Recent advances in large-scale production of monoclonal antibodies and related proteins,” Trends in Biotechnology, vol. 28, no. 5, pp. 253–261, May 2010, doi: 10.1016/J.TIBTECH.2010.02.001.
“Re-use of Protein A Resin: Fouling and Economics.” https://www.biopharminternational.com/view/re-use-protein-resin-fouling-and-economics (accessed Mar. 05, 2022).
C. K. S. Ng, H. Osuna-Sanchez, E. Valéry, E. Sørensen, and D. G. Bracewell, “Design of high productivity antibody capture by protein A chromatography using an integrated experimental and modeling approach,” Journal of Chromatography B, vol. 899, pp. 116–126, Jun. 2012, doi: 10.1016/J.JCHROMB.2012.05.010.
M. Grom, M. Kozorog, S. Caserman, A. Pohar, and B. Likozar, “Protein A affinity chromatography of Chinese hamster ovary (CHO) cell culture broths containing biopharmaceutical monoclonal antibody (mAb): Experiments and mechanistic transport, binding and equilibrium modeling,” Journal of Chromatography B, vol. 1083, pp. 44–56, Apr. 2018, doi: 10.1016/J.JCHROMB.2018.02.032.
“Hollow Fiber Membranes.” https://synderfiltration.com/learning-center/articles/module-configurations-process/hollow-fiber-membranes/ (accessed Dec. 07, 2021).
M. Zhu, “Protein Adsorption and Transport in Novel Chromatographic Anion Exchange and Multimodal Resins,” 2017.
M. Zhu and G. Carta, “Protein adsorption equilibrium and kinetics in multimodal cation exchange resins,” Adsorption, vol. 22, no. 2, pp. 165–179, Feb. 2016, doi: 10.1007/S10450-015-9735-Z/FIGURES/12.
