Project Category: Chemical

Join our presentation
About our project
Gene therapy is a cure for debilitating diseases caused by mutations in the DNA. GetGenics, a contract development and manufacturing organization, produces lentiviral vectors for gene therapy applications using the following production process: upstream bioprocessing that includes seed train and production bioreactor; and downstream purification comprising tangential flow filtration, anion exchange chromatography, and size exclusion chromatography. Each major unit is sized, validated, and optimized for accuracy and economic efficiency. A detailed economic analysis of the process determines its feasibility over a 20-year lifespan with a net present value of $27M, discounted rate of return of 27% and payback period of 10 years. Furthermore, a sensitivity analysis determines that the number of annual production batches, selling price of the product, and dosage levels per disease greatly affect our yearly income. In addition to these analyses, safety, environmental, and social effects are considered in each aspect of the design to make the process robust and effective for servicing the community at large.
Our Process


You can find more information about our major units below!

Seed Train
The seed train increases the number of viable cells taken from the working cell bank (frozen vials of cells) to the desired inoculum density of the production bioreactor through a series of progressively larger vessels.
Production Bioreactor
A three-stage, fed-batch process occurs in the production bioreactor:
- cells are expanded to reach a higher cell concentration for transfection
- transfection reagents are added
- cells begin producing lentiviral vectors
Clarification and Nuclease Treatment
Benzonase treatment to break down large residual DNA and a depth-filtration step to separate HEK293T cells from the bioreactor harvest.
Tangential Flow Filtration (TFF)
A membrane separator operating in crossflow mode to concentrate the LV solution.
Anion Exchange Chromatography (AEX)
Anion exchange chromatography (AEX) is the main protein purification step in our process that uses physicochemical adsorption.
Size Exclusion Chromatography (SEC)
Size exclusion chromatography (SEC) is the polishing step in downstream purification, removing trace amounts of impurities: proteins and DNA (Kamen et al., 2003).
Sterilization and Preservation
The final product is run through a sterile filter of 0.2 µm to meet regulatory requirements. From there, the final product is aliquoted before being stored at -80°C.
Details about our design
HOW OUR DESIGN ADDRESSES PRACTICAL ISSUES
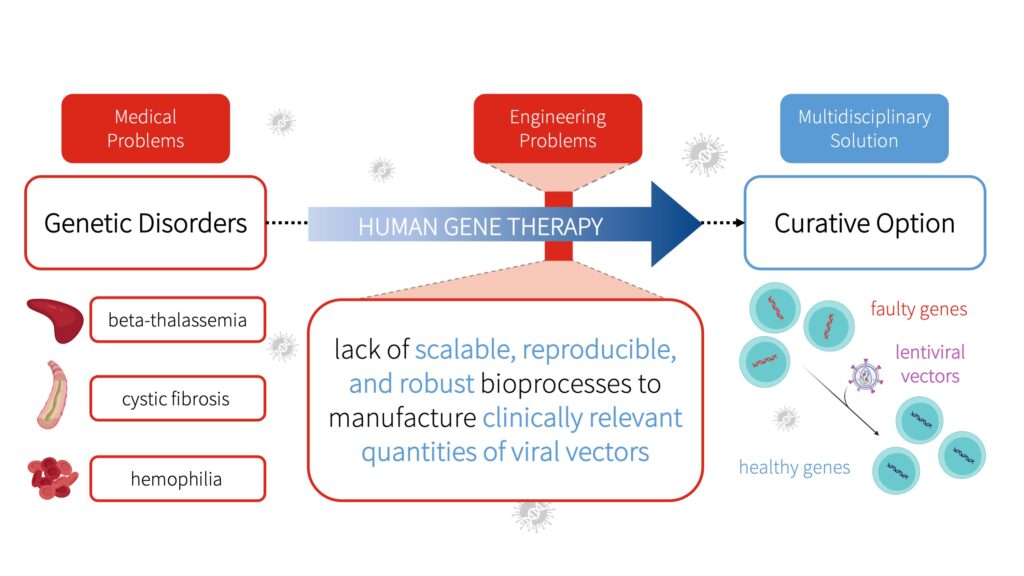
Beta-thalassemia, cystic fibrosis, and hemophilia are examples of debilitating genetic disorders with life-long effects due to alterations in the DNA sequence (Ginter, 2000; Thompson et al., 2018). Unfortunately, current treatment options for these conditions only mitigate the symptoms and are non-curative (Yahya and Alqadhi, 2021).
Gene therapy offers a curative option to improve patients’ quality of life (Maldonado et al., 2020). A common form of gene therapy uses molecular carriers known as lentiviral vectors to add a functional copy of the gene into cells and replace the faulty gene responsible for causing a certain disease (McCarron et al., 2016).
Despite the growing interest in the use of lentiviral vectors for clinical applications, clinical trials have revealed that the lack of scalable, reproducible, and robust bioprocesses to manufacture clinically relevant quantities of lentiviral vectors is a core hindrance in the commercialization and advancement of gene therapies (Merten et al., 2016).
To address these manufacturing challenges and meet future demands, GetGenics have designed a scalable, reproducible, and robust bioprocess to produce lentiviral vectors for human gene therapy applications. By overcoming challenges, GetGenics aims to produce lentiviral vectors required to expedite the clinical translation and innovation of human gene therapy.
WHAT MAKES OUR DESIGN INNOVATIVE
Lentiviral vector production is challenged by the sensitivity of the vectors to pH, shear, buffer osmolarity, freeze-thaw cycles, and thermostability with their low half life of 7-8h at 37℃ (Perry and Rayat, 2021). Further, upstream production of lentiviral vectors is limited by the inability to produce high LV concentration in the product, while downstream purification is limited to low yields (Perry and Rayat, 2021).
To overcome these hurdles, GetGenics developed scalable, high yielding upstream process using suspension-based cultures to overcome scale-up issues associated with adherent cultures. Our process benefits from continuous process monitoring and control to achieve the target product profile for each manufactured lot. We also developed rigorous models to simulate, design, and optimize the seed train and production bioreactor. Indeed, GetGenics used supervised machine learning to accurately fit experimental data from the biotechnology industry and refine the in-silico modelling of the production bioreactor. This tool allowed GetGenics to implement the most optimal scheme possible for the large-scale production of LVs.
Downstream, GetGenics process employed TFF to overcome challenges with shear stress and scalability for concentration (Bandeira et al., 2012). Further, AEX was selected for its short processing time and relatively high yield (Bandeira et al., 2012). SEC was selected for its high purity as a polishing step (Bandeira et al., 2012). Also, GetGenics ordered the downstream units to reduce processing time and costs. TFF first concentrated the large bioreactor harvest which allowed AEX to remove impurities cost-effectively. A subsequent TFF further concentrated the LV solution and allowed SEC to efficiently perform the final purification.
WHAT MAKES OUR DESIGN SOLUTION EFFECTIVE
Upstream Bioprocessing



Scalability
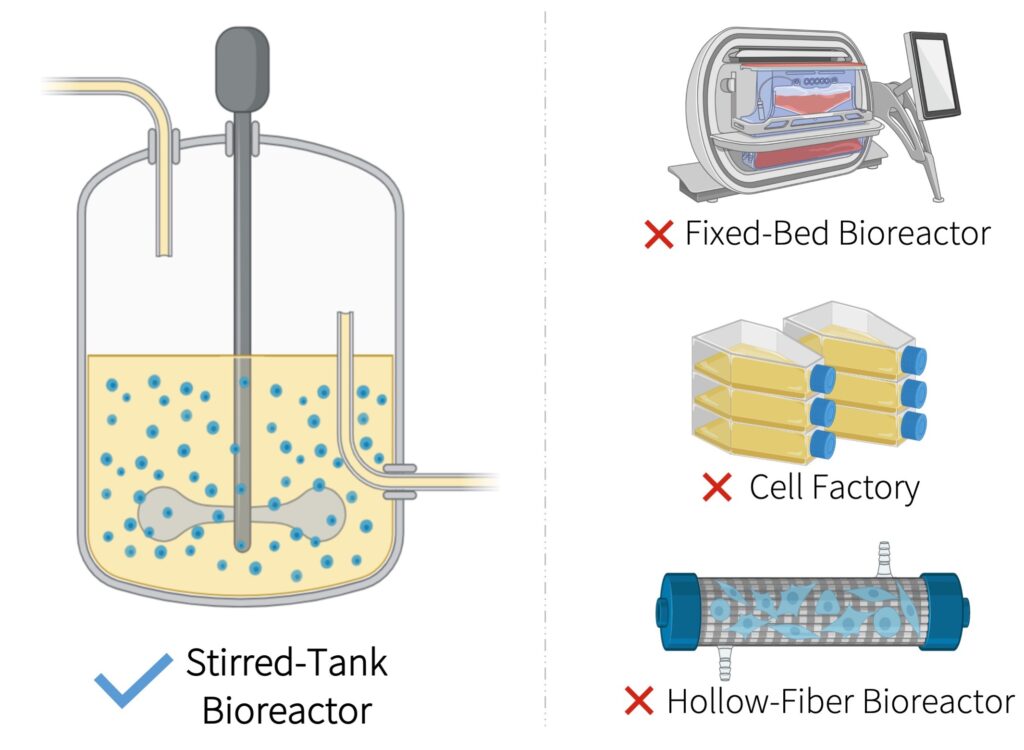
- We produce our main product, lentiviral vectors, in a 500-L stirred-tank bioreactor instead of adherent cell culture technologies that are typically used at the research and development scale
- Our suspension-based process overcomes limited surface area for cell growth and attachment allowing for significantly higher product concentrations in the crude harvest.
- Our scalable process uses a closed production system to reduce manual handling and risk of contamination from open manipulations. It also eliminates labour-intensive work that require skilled operators.
Reproducibility
- We do not use ill-defined components such as animal-derived serum. Instead, we use an animal-derived component-free liquid to support cell growth and expansion.
- Our major units are equipped to monitor and control critical process parameters such as temperature, pH, dissolved oxygen, and agitation rate, which results in a well-controlled extracellular environment, predictable product profile and reduced batch-to-batch variabilities.
Robustness
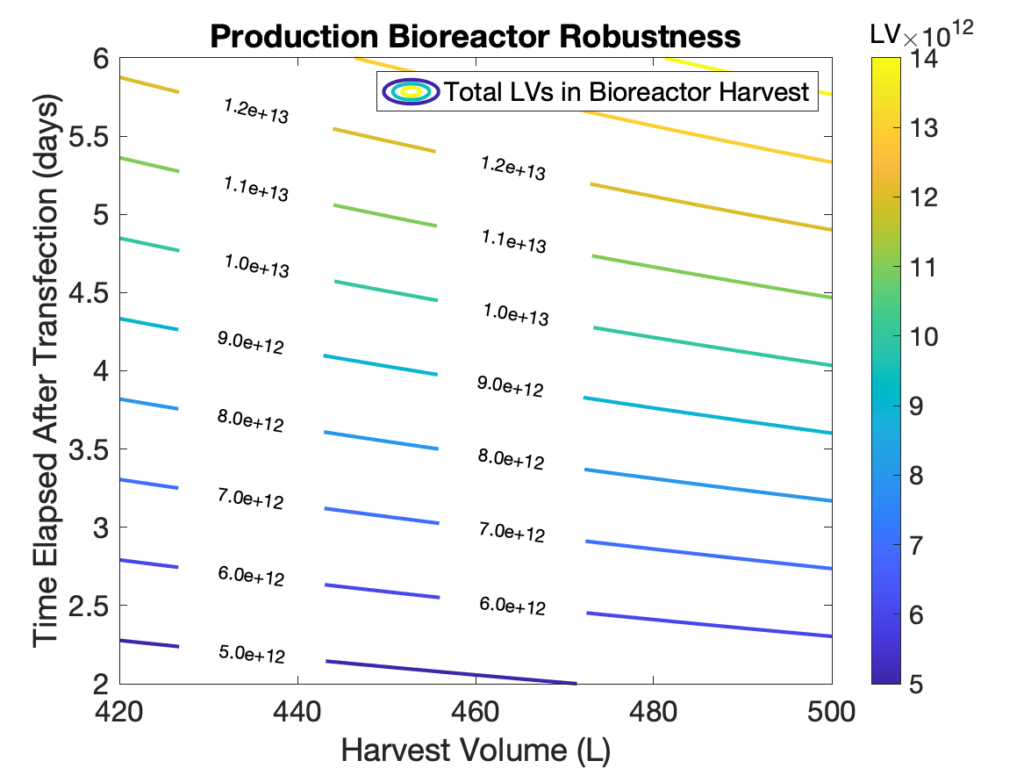
- Our fed-batch bioprocess has demonstrated consistently higher product concentration than other culture methods. Indeed, our upstream process has been optimized and it can produce between 1 to 3×1011 LVs per mL.
- Bioreactor harvest volumes and production times can be easily adjusted to meet client requirements and different target diseases, e.g., upwards of 5×1011 LVs per patient for beta-thalassemia.
Downstream Purification
Design Alternatives
For downstream process, where LVs are purified and concentrated, our team followed general series of steps implemented by the biopharmaceutical industry (Merten et al., 2016). We focused on steps of the process that have several alternatives and these steps were concentration, purification, and polishing. For concentration, we picked tangential flow filtration (TFF) and for purification step, we picked anion exchange chromatography (AEX). Note that for polishing step, size exclusion chromatography (SEC) was used due to its gentle and straightforward purification with LV recovery ranging from 70% to 80%, while removing more than 99% of trace impurities (McCarron et al., 2016).

Process Robustness
Our downstream units can purify variety of vector titers and supernatant solution from the production bioreactor. These units have been simulated to verify robustness and have proven to be time efficient where it takes roughly 2 days to achieve our final product.
HOW WE VALIDATED OUR DESIGN SOLUTION
Each major unit was validated using secondary simulations. These secondary simulations were independent from the initial sizing models and utilized a different set of governing equations and correlations. Further details on each unit’s validation can be found in “Learn more about our major units” below.
FEASIBILITY OF OUR DESIGN SOLUTION
Our process utilizes commercially available equipment and reagents for our scalable, robust production of viral vectors. Additionally, our product is profitable with an NPV of $27M (further economic details below). As such, our process is a feasible way to increase viral vector manufacturing capacity.
Economics
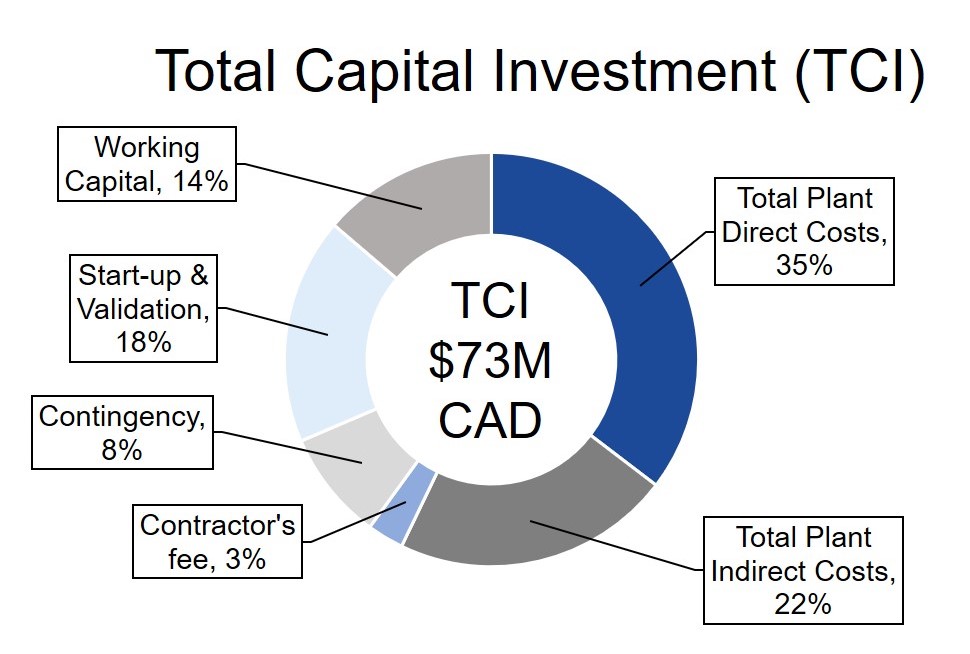
GetGenics designed an economically feasible process with a project lifetime of 22 years and net present value (NPV) of $27M. GetGenics produces 10 batches annually and has a selling point of $450k per patient. Each batch treats 10 patients, for an annual revenue of $45M. Please see the figures below for a breakdown of our fixed capital investment and annual operating expenses (OPEX), along with other financial indicators.
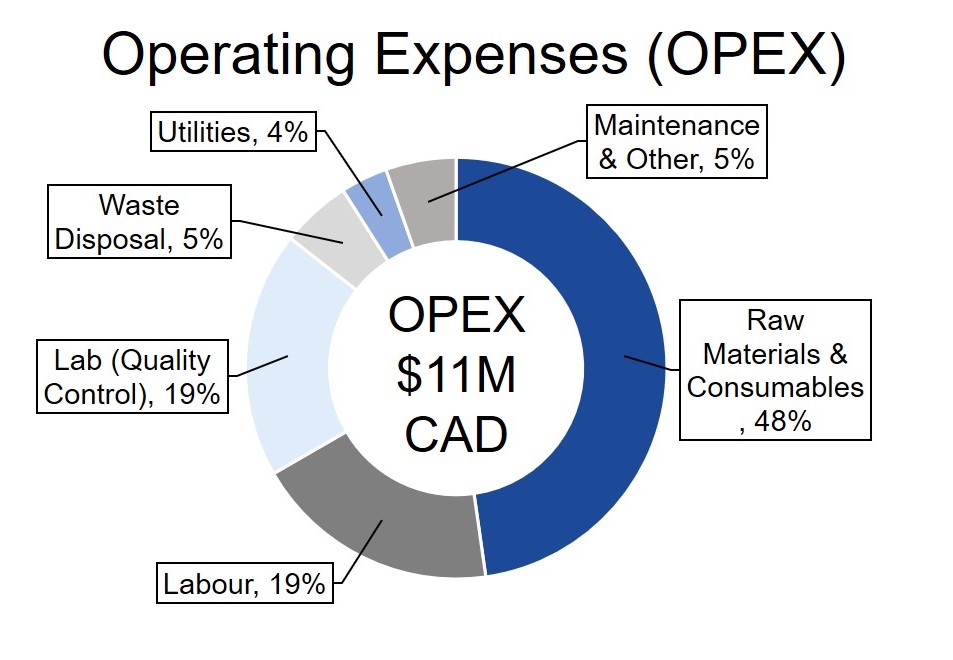

Sensitivity Analysis
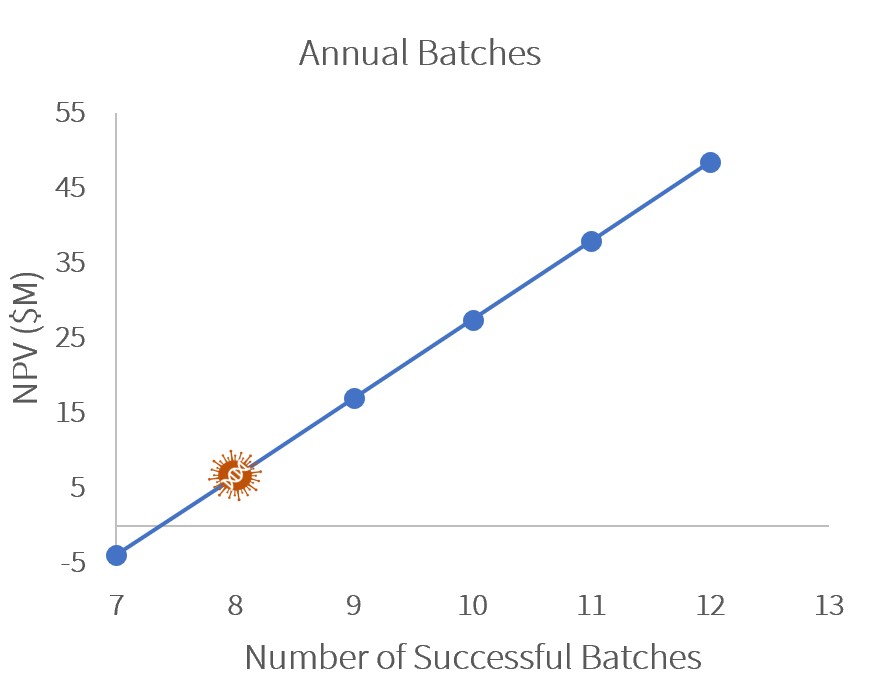
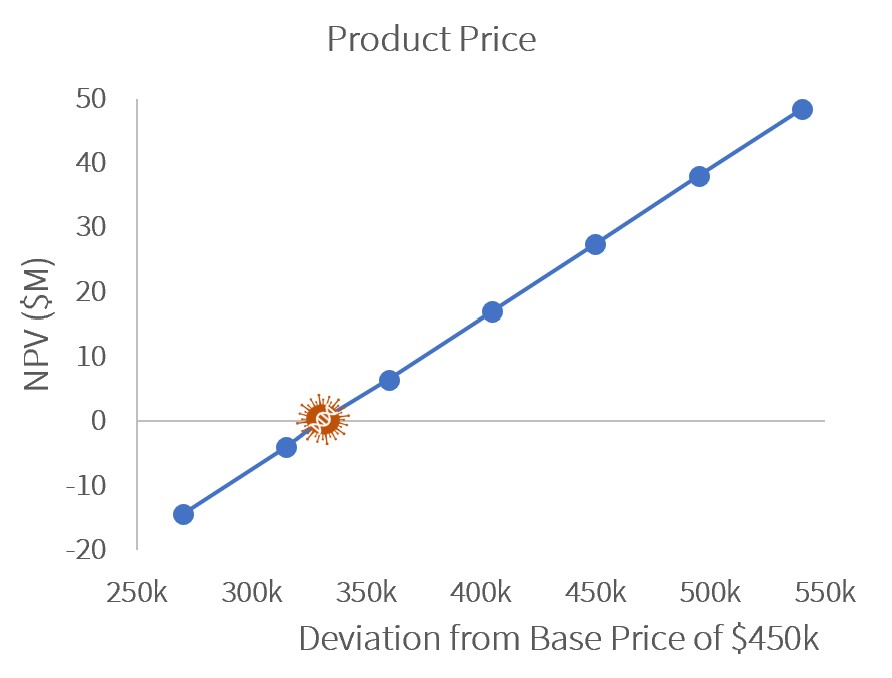
A sensitivity analysis showed that to remain profitable (NPV>$0), GetGenics would need to produce at least 8 annual batches or sell our product at $330k/patient.
Facility details


- accessible facility located near the airport
- dedicated suites for cell growth and product purification
- emphasis on segregation to prevent contamination
- segregation is accomplished by process design, environmental controls, and procedural segregation
Safety Considerations

Safe vectors
Third generation vectors are used because of their proven safety in clinical trials (Negre et al., 2014)
Containment
Viruses are contained in a closed system during manufacturing.
Cleaning validation
All equipment is tested for contaminants and adventitious agents before processing the next batch (Raj, 2014).
Disinfecting
Equipment is disinfected with a sterilization in place system to ensure sterility.
Batch testing
Prior to being released to the public, the final product undergoes extensive batch release testing to check for impurities and concentration levels (Gá et al., 2018).
Standard operating procedures (SOPs)
SOPs ensure trained personnel are protected from potential hazards.
Regulations
Health Canada’s regulations regarding lentiviral vectors are followed throughout the production timeline (Chisholm et al., 2019).
Product recall
In the case anything is wrong with a released batch, a Class I product recall procedure will put into place (Vvss et al., 2020).
Personal protective equipment (PPE)
Staff will wear PPE to further protect their safety.
Good manufacturing practice (GMP) compliant facility
A GMP compliant facility with bidirectional flow will manufacture the viruses in a safe and effective manner (Halkjaer-Knudsen, 2007)
Learn more about our major units
Upstream Bioprocessing
Cell expansion in the Seed Train
The seed train increases the number of viable cells to the desired inoculum density of the production bioreactor. Cells are grown in suspension and transferred to the next larger stage when a density of 2.5×106 cells/mL is reached. As shown below, there is a total of 5 stages in the seed train starting with three shake flasks (0.05L, 0.25L, and 1L) and two rocking bioreactors (5L and 20L).
Validation and Optimization
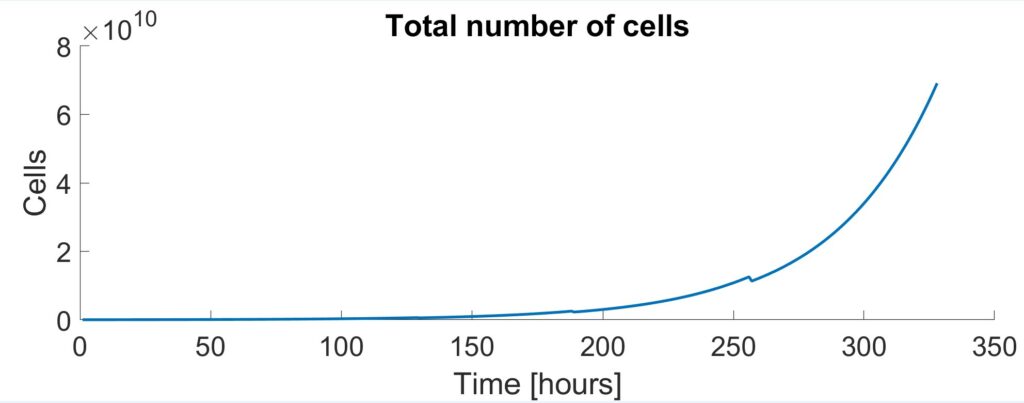
A modified version of Monod Kinetics (Kern et al., 2016) was initially used to model the cell densities and concentrations of the metabolites within the cell culture. Cell concentrations dictated the size of each vessel and the amount of time needed for each stage.
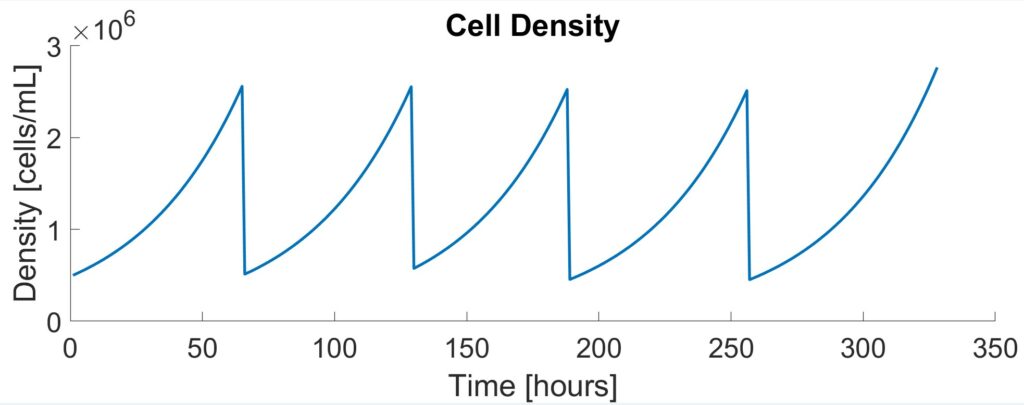
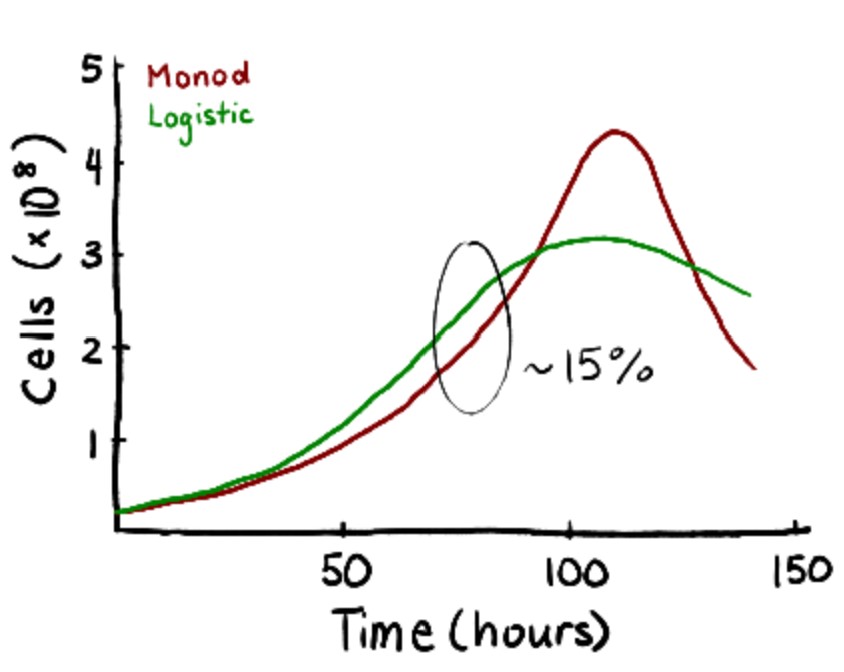
To validate the initial Monod results, experimental data from Patel et al. were fitted to logistic equations. While less comprehensive than Monod kinetics, the logistic model demonstrates the exponential rising and falling of cell concentrations during each stage of the seed train. To validate the model, the time it took for the cells to reach passaging density of 2.5×106 cells/mL was compared. There was a 13.3% difference between the two models (60 hours versus 50 hours) which was close enough to validate.


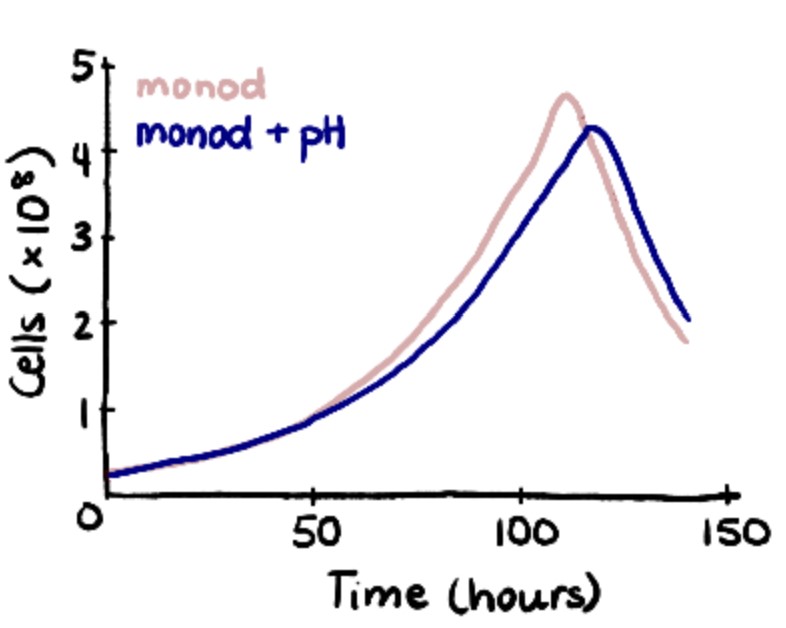
To further enhance the model, the effects of pH were included. ODEs proposed by Gadgil et al. were implemented into the Monod Kinetic model to account for the uncontrolled pH in the first three shake flask stages. This caused cell growth to slow down and increased the run from 314 hours to 328 hours.
Production of viral vectors in the Production Bioreactor
The production bioreactor is the final vessel in which cells are grown, maintained, and manipulated in a controlled environment for lentiviral vector manufacturing. It uses engineering process controls to maintain pH, temperature, dissolved oxygen levels, agitation rate, and pressure inside the vessel.
To achieve the production target, the production bioreactor can be broadly divided into three-stages as shown below:


Transfection uses polyethyleneimine (PEI) and a four-plasmid system to equip cells with packaging constructs required to successfully produce lentiviral vector particles containing the therapeutic gene of interest. A schematic of the transfection process is shown below:

Validation and Optimization
To simulate the bioreactor, experimental data from the industry (Patel et al., 2020) was fitted using supervised machine learning. In brief, alternative cell culture models (Cruz et al., 2000; Frahm, 2014; Karra et al., 2011; Kern et al., 2016) based on the Monod equation that use a system of ordinary differential equations (ODEs) were first considered. Using ODE parameters for mammalian cells found in literature, the most parsimonious model that describes the trends in cell, nutrients, metabolite, and LV concentrations was selected. The model was trained to refine the parameters that appear in the system of ODEs. Then, the model’s robustness was demonstrated by showing goodness of fit using independent validation sets. The simulations results are in agreement with typical ranges reported in literature (Comisel et al., 2020; Perry and Rayat, 2021) and thus, validates our modelling efforts. To optimize the performance of the bioreactor, the effects of changing the feeding regime were simulated. The impact of critical process parameters including pH, temperature, dissolved oxygen, and agitation rate were also assessed. The simulation results of with optimized conditions are summarized below.



Downstream Purification
Concentration with Tangential Flow Filtration
TFF is the main concentration step in the downstream process, removing 80% of residual DNA, 80% of protein, and 99% of benzonase while concentrating the LV solution by 20-25-fold (Gousseinov and Pattnaik, 2014; Loewe et al., 2019). TFF is a membrane filter separating on the principle of size exclusion. Small impurities flow through the membrane while the large LVs flow along the membrane forming the permeate and retentate streams, respectively. TFF differs from depth-filtration in that the feed flows tangential to the membrane. Tangential flow reduces the buildup of solutes on the membrane surface and results in a higher permeate flux (Lutz, 2015).
Validation and Optimization
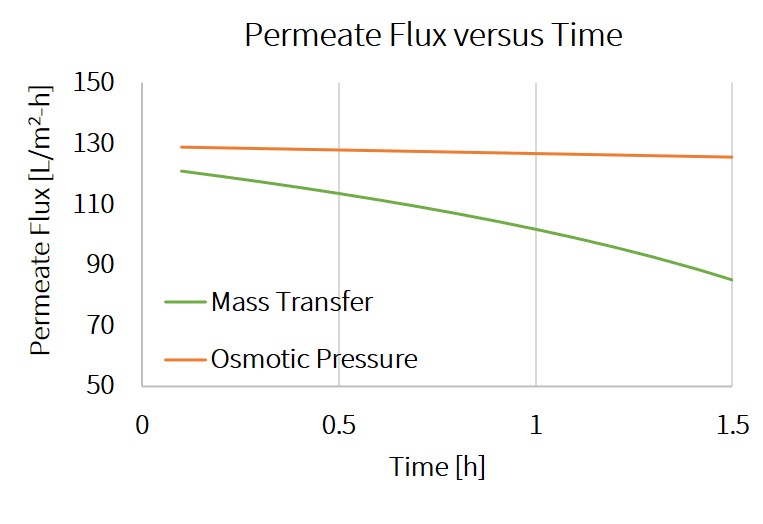
To model TFF operation, two independent models were used. Gel polarization is a mass transfer model using mass transfer coefficient and concentrations within the TFF to model permeate flux (Lutz, 2015). An osmotic pressure model focusses on the concentration of solutes on the retentate and permeate side of the membrane to model the permeate flux decline (Thiess et al., 2017). The permeate flux is the amount of solution leaving the membrane and declines during operation due to buildup of solutes. Both models along with experimental data were within the same magnitude of each other and thus validated the simulation model.

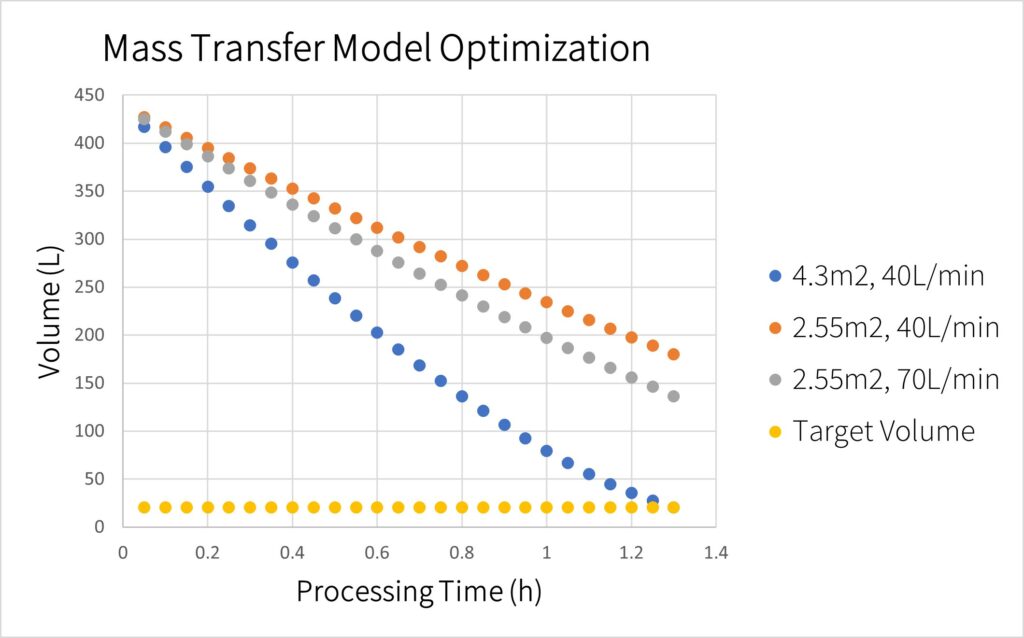
For TFF two main parameters flow and membrane area are manipulated to increase permeate flux. Increasing membrane area provides more surface for the impurities and solution to flow through at the expense of a costlier membrane. Increasing flow rate results in a larger mass transfer coefficient leading to a higher permeate flux at the expense of increasing shear stress on the LVs. As can be seen in the figure, increasing membrane area had a more profound impact on permeate flux than increasing flow rate.
Protein Purification with Anion Exchange Chromatography
AEX chromatography is the main protein purification unit in our downstream process that removes 99.85% of proteins and 15.79% of DNA (Merten et al., 2011). At a pH of 8.0, LVs are negatively charged and it binds to the positively charged membrane of the column, allowing for effective separation (Bandeira et al., 2012). Thus, AEX is a physicochemical adsorption unit.

Validation and Optimization
To model and validate the AEX unit, we used two independent simulations. The first simulation is based on the Klinkenberg equation that models non-ideal fixed bed adsorption using linear driving force model (Seader et al., 2019). The second simulation used two partial differential equations (PDEs) that are based on mass balance equation and uses Langmuir isotherm (Gu, 2015). The second simulation software, Chromulator, was kindly provided by Dr. Tingyue Gu (Gu, 2015).

From optimization using both Klinkenberg equation and Chromulator software, the AEX column with a volume of 260 ml will be packed with POROS D50 resin. With this set-up, we were able to achieve vector recovery of 77% with a runtime of 40 min. To validate our results, we used literature findings. For example, Merten et al., ran a feed volume of 48 liters with a resulting elution volume of 2 litres (Merten et al., 2011). From the elution volume, the bed volume was around 400 ml, since five column volumes of elution buffer is typically used to unbind the vectors (Yamada et al., 2003). Moreover, Valkama et al. used a 400 ml chromatography column to purify 180 liters of bioreactor harvest that went through clarification and TFF concentration units (Valkama et al., 2020).
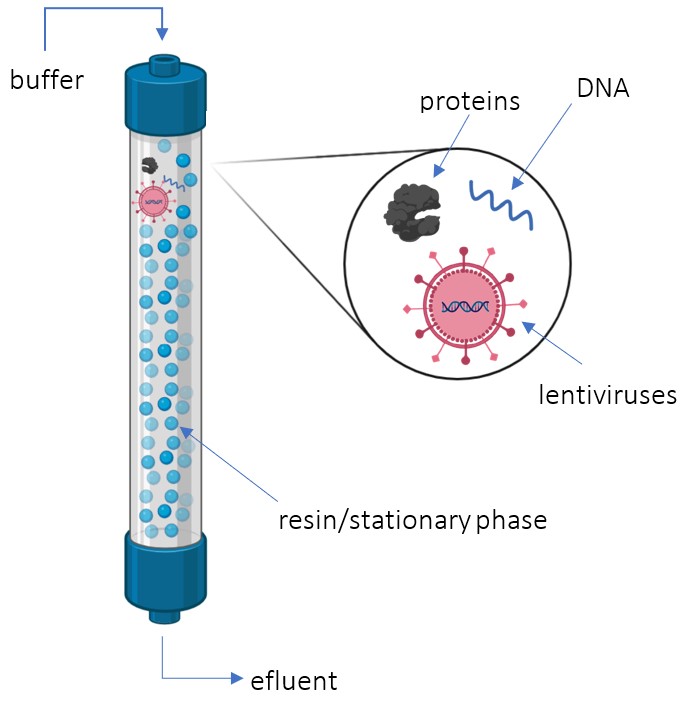
Polishing with Size Exclusion Chromatography
SEC takes in a feed with the desired product: lentiviruses (LVs), impurities: trace amounts of proteins and DNA, as well as constantly buffer (Kamen et al., 2003). The column separates the LVs from impurities based on size (Kamen et al., 2003). The lentiviruses are much larger than the resin’s pores and therefore do not enter the column while the impurities do (Segura et al., 2006). As a result, the desired product exits the column first, while impurities are eluted later. Overall, the SEC acts as the final polishing step in downstream purification, removing trace amounts of impurities: proteins and DNA based on size (Kamen et al., 2003).
Validation and Optimization
The SEC column was sized based on two, independent models. The first model simulated impurities in MATLAB based on a plug flow assumption with a system of two partial differential equations (Sejergaard et al., 2017). Based on the simplified model, a column length of 0.40 m and outer diameter 0.05 m could remove 98.71% of impurities. To validate the original design, secondary simulations were completed in the Chromulator software provided by Dr. Tingyue Gu (Gu, 2015). This secondary model used a different and more accurate pair of partial differential equations (PDEs). Using original design parameters, the simulations determined the column removes 85.72% of impurities, resulting in a 13.16% error between the two models.
Once validated, the column length, diameter, and resin were optimized using Chromulator. It was found that the highest purity levels that could be achieved were 92.85% using a column length of 0.60 m and outer diameter of 0.05 m, as shown in the figure. These dimensions matched those to the commercially available unit of XK 50/60. Additionally, it was found that column resin had negligible effects on purity and price.

Meet our team members

Jessica 
Huneza 
Michidmaral 
Phillip 
Thristan
Jessica May Corpuz
Jessica is a final year chemical engineering student with a biomedical engineering specialization. She has experience in the plastics industry through her internship at NOVA Chemicals and research experience in cartilage and intervertebral disc degeneration and regeneration. She has strong interest in R&D and hopes to follow this career path.
Huneza Nadeem
Huneza is a final year chemical engineering student, specialising in biomedical engineering. She worked at ABB’s corporate research centre for a year in Switzerland, where she further developed her data analysis and image processing skills. She is passionate about creating interdisciplinary solutions for problems in medicine.
Phillip Scheck
Phillip is a chemical engineering student in his final year with work experience in process engineering through his internship with Canadian Natural Resources Limited (CNRL). Internship provided him opportunities to work on HAZOP studies, design work, and optimization of a primary upgrader in Fort McMurray. Phillip is devoted to safety and completing work with due diligence and integrity.
Thristan Paulo Taberna
Thristan is in his final year of chemical/biomedical engineering at the University of Calgary. He completed his internships at BioMarin Pharmaceutical (San Francisco Bay Area) and STEMCELL Technologies (Vancouver). As an aspiring biomedical engineer, Thristan aims to contribute to the renaissance in regenerative medicine technologies. His research interest lies in applying biochemical engineering principles to develop robust, scalable bioprocesses for the production of high-quality therapeutics from stem cells.

Michidmaral Otgondavaa
Maral is a fifth-year chemical engineering student with a biomedical engineering specialization. She has research experience in membrane water filtration and medical imaging, and internship experience with Crescent Point Energy Corp. She has strong interest in sustainability, project management, and machine learning.

Acknowledgements
We owe a deep sense of gratitude to our capstone supervisor, Dr. Michael Scott Kallos, for his constant guidance, words of encouragement, and enthusiasm. Similarly, we would like to profusely thank our course instructor, Dr. Hector De la Hoz Siegler, for taking the time to teach, guide, and motivate us throughout this semester. Additionally, we are extremely thankful for our industry partners, Dr. Krishna Panchalingam and Dr. Tylor Walsh, for their keen industrial insight into the lentiviral vector production process.


References
Images were created using BioRender
References
Bandeira V, Peixoto C, Rodrigues AF, Cruz PE, Alves PM, Coroadinha AS, Carrondo MJT. 2012. Downstream processing of lentiviral vectors: releasing bottlenecks. Hum. Gene Ther. Methods 23:255–263.
Chisholm J, Ruff C, Viswanathan S. 2019. Current state of Health Canada regulation for cellular and gene therapy products: potential cures on the horizon. Cytotherapy. Elsevier B.V.
Comisel R-M, Kara B, Fiesser FH, Farid SS. 2020. Lentiviral vector bioprocess economics for cell and gene therapy commercialisation. Biochem. Eng. J.:107868. https://linkinghub.elsevier.com/retrieve/pii/S1369703X20304228.
Cruz PE, Almeida JS, Murphy PN, Moreira JL, Carrondo MJT. 2000. Modeling Retrovirus Production for Gene Therapy. 1. Determination of Optimal Bioreaction Mode and Harvest Strategy. Biotechnol. Prog. 16:213–221. https://doi.org/10.1021/bp9901466.
Frahm B. 2014. Seed Train Optimization for Cell Culture BT – Animal Cell Biotechnology: Methods and Protocols. In: Pörtner, R, editor. Totowa, NJ: Humana Press, pp. 355–367. https://doi.org/10.1007/978-1-62703-733-4_22.
Gá C, Affleck V, Stoll EA. 2018. Manufacture of Third-Generation Lentivirus for Preclinical Use, with Process Development Considerations for Translation to Good Manufacturing Practice. Hum. Gene Ther. Methods 29:1–15.
Ginter EK. 2000. [Gene therapy of hereditary diseases]. Vopr. Med. Khim. 46:265–278.
Gousseinov E, Pattnaik P. 2014. Nucleic Acid Impurity Reduction in Viral Vaccine Manufacturing. Bioprocess Int. https://bioprocessintl.com/upstream-processing/assays/nucleic-acid-impurity-reduction-in-viral-vaccine-manufacturing-349787/.
Gu T. 2015. Chromulator software. https://people.ohio.edu/gu/CHROM/index_chrom.html.
Halkjaer-Knudsen V. 2007. Designing a Facility with Both Good Manufacturing Practice (GMP) and Biosafety in Mind: Synergies and Conflicts. Appl. Biosaf. 12:7–16. https://journals.sagepub.com/doi/abs/10.1177/153567600701200102.
Kamen A, Jesus C, Transfiguracion J. 2003. Size-Exclusion Chromatography Purification of High-Titer Vesicular G Glycoprotein-Pseudotyped Retrovectors for Cell and Gene Therapy Applications. Hum. Gene Ther. 14:1139–1153.
Karra S, Sager B, Nazmul Karim M. 2011. A Comprehensive Multi-Scale Modeling of Heterogeneities in Mammalian Cell Culture Processes. In: Pistikopoulos, EN, Georgiadis, MC, Kokossis, ACBT-CACE, editors. 21 Eur. Symp. Comput. Aided Process Eng. Elsevier, Vol. 29, pp. 1311–1315. http://www.sciencedirect.com/science/article/pii/B9780444542984500416.
Kern S, Platas-Barradas O, Pörtner R, Frahm B. 2016. Model-based strategy for cell culture seed train layout verified at lab scale. Cytotechnology 68:1019–1032. /pmc/articles/PMC4960151/?report=abstract.
Loewe D, Grein TA, Dieken H, Weidner T, Salzig D, Czermak P. 2019. Tangential Flow Filtration for the Concentration of Oncolytic Measles Virus: The Influence of Filter Properties and the Cell Culture Medium. J. Memb. Sci. 9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6950090/.
Lutz H. 2015. Ultrafiltration for Bioprocessing 1st ed. Woodhead Publishing 244 p.
Maldonado R, Jalil S, Wartiovaara K. 2020. Curative gene therapies for rare diseases. J. Community Genet. https://doi.org/10.1007/s12687-020-00480-6.
McCarron A, Donnelley M, McIntyre C, Parsons D. 2016. Challenges of up-scaling lentivirus production and processing. J. Biotechnol. 240:23–30. http://dx.doi.org/10.1016/j.jbiotec.2016.10.016.
Merten O-W, Hebben M, Bovolenta C. 2016. Production of lentiviral vectors. Mol. Ther. Methods Clin. Dev. 3:16017.
Merten OW, Charrier S, Laroudie N, Fauchille S, Dugué C, Jenny C, Audit M, Zanta-Boussif MA, Chautard H, Radrizzani M, Vallanti G, Naldini L, Noguiez-Hellin P, Galy A. 2011. Large-scale manufacture and characterization of a lentiviral vector produced for clinical Ex vivo gene therapy application. Hum. Gene Ther. 22:343–356.
Negre O, Bartholomae C, Beuzard Y, Cavazzana M, Christiansen L, Courne C, Deichmann A, Denaro M, Dreuzy E, Finer M, Fronza R, Gillet-Legrand B, Joubert C, Kutner R, Leboulch P, Maouche L, Paulard A, Pierciey F, Rothe M, Ryu B, Schmidt M, Kalle C, Payen E, Veres G. 2014. Preclinical Evaluation of Efficacy and Safety of an Improved Lentiviral Vector for the Treatment of β-Thalassemia and Sickle Cell Disease. Curr. Gene Ther. 15:64–81. /pmc/articles/PMC4440358/?report=abstract.
Patel S, Fong E, George H. 2020. Considerations for Bioreactor Process Development and Scale-Up for Transient Transfection-Based Lentivirus Production in Suspension. BioProcess Online:1–8. https://www.bioprocessonline.com/doc/considerations-for-bioreactor-process-development-and-scale-up-for-transient-transfection-based-lentivirus-production-in-suspension-0001.
Perry C, Rayat ACME. 2021. Lentiviral Vector Bioprocessing. Viruses .
Raj A. 2014. Cleaning Validation in Pharmaceutical Industries. J. Atoms Mol. 4:779–783.
Seader JD, Henley EJ, Roper K. 2019. Separation process principles with applications using process simulators. Ed. Linda Ratts, Mary Sullivan, Adria Giattino 4th ed. New York: John Wiley and Sons Inc. 470–488 p.
Segura M de LM, Kamen A, Garnier A. 2006. Downstream processing of oncoretroviral and lentiviral gene therapy vectors. Biotechnol. Adv. 24:321–337.
Sejergaard L, Ahmadian H, Hansen TB, Staby A, Hansen EB. 2017. Model-Based Process Development in the Biopharmaceutical Industry. In: . Prep. Chromatogr. Sep. Proteins. Hoboken, NJ, USA: John Wiley & Sons, Inc., pp. 429–455. http://doi.wiley.com/10.1002/9781119031116.ch14.
Thiess H, Leuthold M, Grummert U, Strube J. 2017. Module design for ultrafiltration in biotechnology: Hydraulic analysis and statistical modeling. J. Memb. Sci. 540:440–453.
Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil J-A, Hongeng S, Magrin E, Schiller GJ, Payen E, Semeraro M, Moshous D, Lefrere F, Puy H, Bourget P, Magnani A, Caccavelli L, Diana J-S, Suarez F, Monpoux F, Brousse V, Poirot C, Brouzes C, Meritet J-F, Pondarré C, Beuzard Y, Chrétien S, Lefebvre T, Teachey DT, Anurathapan U, Ho PJ, von Kalle C, Kletzel M, Vichinsky E, Soni S, Veres G, Negre O, Ross RW, Davidson D, Petrusich A, Sandler L, Asmal M, Hermine O, De Montalembert M, Hacein-Bey-Abina S, Blanche S, Leboulch P, Cavazzana M. 2018. Gene Therapy in Patients with Transfusion-Dependent β-Thalassemia. N. Engl. J. Med. 378:1479–1493. https://doi.org/10.1056/NEJMoa1705342.
Valkama AJ, Oruetxebarria I, Lipponen EM, Leinonen HM, Käyhty P, Hynynen H, Turkki V, Malinen J, Miinalainen T, Heikura T, Parker NR, Ylä-Herttuala S, Lesch HP. 2020. Development of Large-Scale Downstream Processing for Lentiviral Vectors. Mol. Ther. – Methods Clin. Dev. 17:717–730. https://doi.org/10.1016/j.omtm.2020.03.025.
Vvss R, Veluchuri J, Adhikari S, Indukuri H. 2020. An Overview on Pharmaceutical Drug Recalls:16–22.
Yahya EB, Alqadhi AM. 2021. Recent trends in cancer therapy: A review on the current state of gene delivery. Life Sci. 269. https://www-sciencedirect-com.ezproxy.lib.ucalgary.ca/science/article/pii/S0024320521000722.
Yamada K, McCarty DM, Madden VJ, Walsh CE. 2003. Lentivirus vector purification using anion exchange HPLC leads to improved gene transfer. Biotechniques 34:1074-1078,1080.
