About our project
Following the Paris Agreement, more than 175 nations aim to achieve zero net emissions of greenhouse gases in the second half of the century. One method to mitigate emissions is to directly capture CO2 from the atmosphere using Direct Air Capture (DAC) technology. DAC offers a unique solution by removing CO2 from sources such as airplanes and cars that account for 60% of total CO2 emissions. In addition to carbon removal technology, there is increasing interest in utilizing CO2 as raw material for several processes.
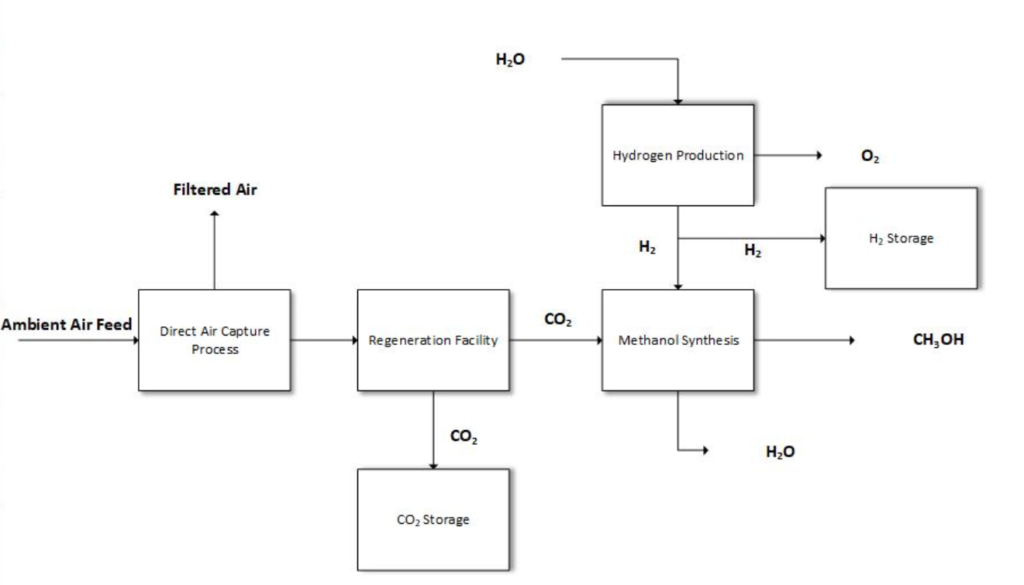
As such, the objective of our project is to produce methanol by using direct hydrogenation of captured CO2. After capturing atmospheric CO2, we will obtain 98% purity CO2 after processing. Pure hydrogen from Proton Exchange Membrane (PEM) electrolysis will also be a main feedstock to produce our methanol. With an importance on the efficiency of our process, PEM electrolysis will supply our hydrogen in our reaction.
Meet our team members 🙂

Braedon Neufeld
Graduating, 5th year Chemical Engineering student at the University of Calgary. Completed a 12-Month Internship at Imperial Oil Ltd as a Risk and Safety Engineering Co-op. Hobbies include fitness and cooking.

Bernice Yeung
Graduating, 5th year Chemical Engineering student at the University of Calgary. Completed a 16-Month Internship with the Department of National Defence as a Project Assistant for Real Property Operations North. Bernice aims to return to the public service upon graduation.

Kevin Mesa
Graduating, 5th year Chemical Engineering student at the University of Calgary. Completed a 13-Month Internship at Imperial Oil as a Project Execution Intern for Canada Fuels Operation (Midstream). Hobbies include basketball, fitness and cooking. Big fan of dogs and cats.

Sojeong (Jenna) Yun
Graduating, 5th year Chemical Engineering student at the University of Calgary. Completed a 12-Month Internship at Husky Energy as a Engineering Co-op for Pipeline integrity and integrity governance team. Loves dogs and cats. Hobbies are drawing.

Murtaza Sadat
Graduating, 5th year Chemical Engineering student at the University of Calgary. Completed a 12-Month Internship at Montrose Environmental as a Field engineer fugitive emission specialist. Hobbies include Muai Thai, and basketball.
Details about our design
HOW OUR DESIGN ADDRESSES PRACTICAL ISSUES
With rising concentrations of CO2 in the atmosphere, it is pertinent to develop carbon capture technology to mitigate climate change for future generations. By utilizing Direct Air Capture of CO2, we are combating the challenges of CO2 removal at point source from origins such as airplanes and cars. An additional benefit to our process is utilizing captured CO2 in sequestration (carbon underground storage) and direct hydrogenation to Methanol, a high energy-density, versatile fuel that can be used in several applications.
WHAT MAKES OUR DESIGN INNOVATIVE
Direct Air Capture (DAC) is a unique, innovative technology receiving recognition, research and global acknowledgment from governments and related industries. DAC captures CO2 directly from atmospheric air and then converts it to usable and pure CO2 (98%). This is done by reacting a liquid capture solution with atmospheric air and then processing it to a regeneration facility where the carbonate solution undergoes causticization. After it is then fed to a calciner (slaker) where it is heated to 900°C which produces the highly concentrated CO2.
In contrast to conventional methanol production using syngas or coal feedstocks, our design pairs unconventional upstream carbon capture with downstream PEM units for methanol synthesis, with the goal of furthering technological development in the production of clean alternative fuels. PEM technology is an innovative system that generates sufficient amounts of hydrogen using renewable energy. Unlike other methods PEM electrolysis allows us to produce pure clean hydrogen with significantly reduced emissions, maintaining our goal of limiting CO2 emissions. The PEM process is expected to be powered by renewable energy sources such as wind and solar.
Source: Carbon Engineering. Link: https://youtu.be/PraDOusY87g
WHAT MAKES OUR DESIGN SOLUTION EFFECTIVE
Our DAC process is conceptually designed to capture a major amount of CO2 from the atmosphere at 1.3 Megatons per year, this design closely follows the most recent and well-developed research from a Canadian Company called Carbon Engineering. Our extensive design research in this new technology and implementation plan has prepared a CO2 capturing unit with a promising production level. Based on the engineering and chemical parameters, DAC is an effective solution to fighting against climate change especially paired with renewable energy sources.
Our solution doesn’t stop there. We will be utilizing our captured CO2 to produce a valuable, high energy-density fuel. Methanol can be used in several applications, such as an intermediate for producing hundreds of chemicals. To produce methanol, we will be using PEM electrolysis to supply the hydrogen needed. PEM technology is sought for its high current and pressure operation in addition to its compact design. The membrane in our system is the backbone of our PEM cell. These membranes have unique properties such as high strength, high efficiency and high oxidative stability, dimensionally stabile with change of temperatures, good durability and high proton conductivity. Excess CO2 captured will be sequestrated to maximize the environmental benefits of our process.
HOW WE VALIDATED OUR DESIGN SOLUTION
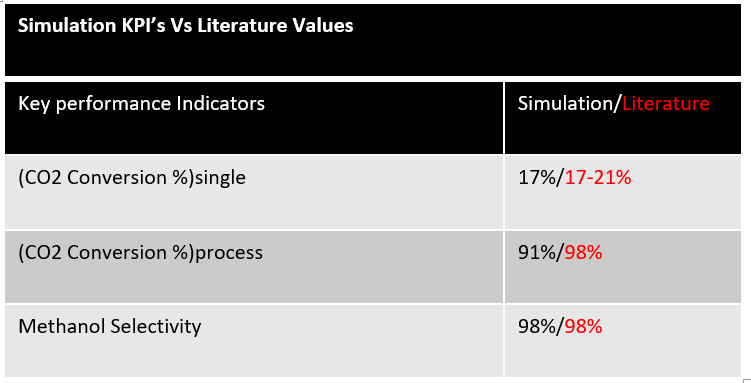
A full simulation of our carbon dioxide to methanol process was built in Symmetry; this includes all major pieces of equipment, such as the plug flow reactor and distillation column. The fundamentals of our design were based on literature data before it was scaled to meet our desired production rate. Scaling was achieved with confirmation that thermodynamic and kinetic results mirrored literature values.
Methanol Synthesis Reactor
The main reaction taking place for Methanol synthesis are:
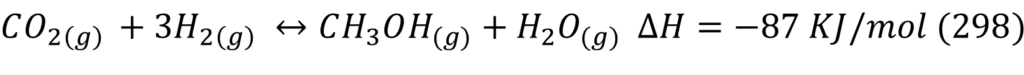
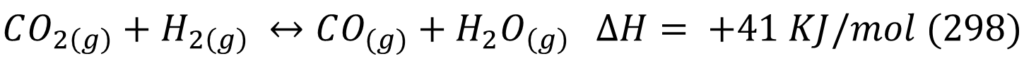
Reactor design was started by looking at thermodynamic and kinetic literature data on CO2 hydrogenation reactions. It was important to ensure the right temperature and pressure were chosen for the reactor operation because the rWGS side reaction consumes valuable reactant and produces water which can poison and deactivate the catalyst.
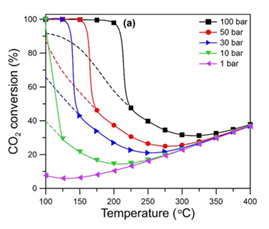
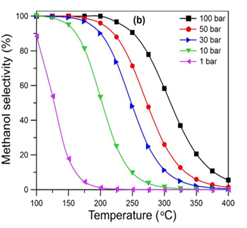
The unwanted side reaction is endothermic while the hydrogenation reaction is exothermic, thus high temperatures are favourable. Additionally, following Le Chatliers principle, the hydrogenation reaction is pushed to the products at high pressures. Therefore Medium-High Temperatures and High Pressures are ideal. These conditions can be further justified by looking at alternative Laboratory data on hydrogenation reactions as seen in the charts.
Once the operating conditions were chosen a bench scale simulation was created to test our reaction rates and emulate bench scale experimental data. As you can see, using literature values, our bench scale reactor on the left is comparable and simulates as expected telling us our rates are correct, the operating conditions are favorable, and the reactor can be scaled up for production.
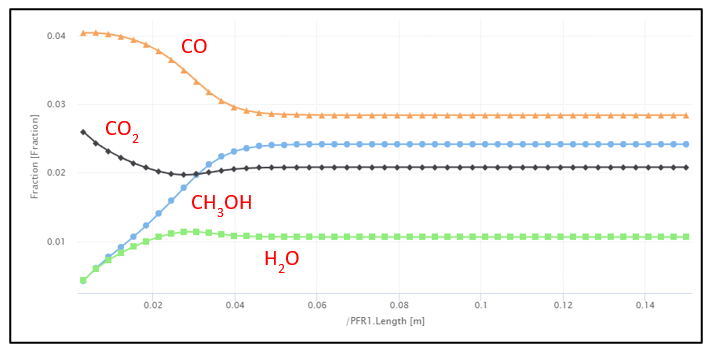
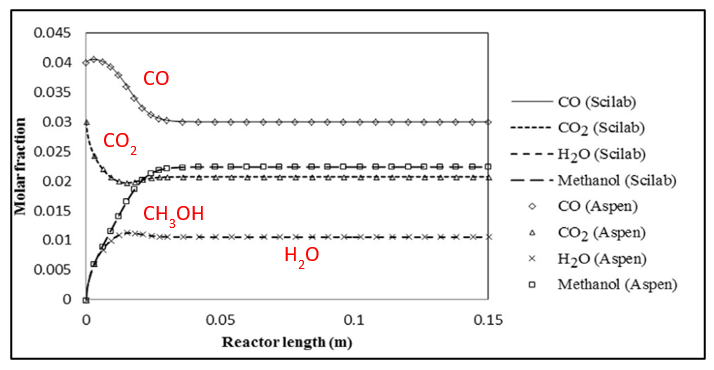
Direct Air Capture (DAC) and Proton Exchange Membrane (PEM) Validation
As mentioned beforehand, the design of the DAC process is rather closely following the most recent and well-developed research from a Canadian company called Carbon Engineering. They have recently built their pilot plant (small scale) and are continuously monitoring it for improvements in the future. They have publicly released many academic papers of their DAC findings/studies including futuristic DAC processes that are scaled up to capture 1 Megaton of CO2 a year. Our rigorous design research in this new technology and implementation plan has prepared a CO2 capturing unit with a satisfying production level. We’ve also validated this design by incorporating and comparing other academic papers reviewing the same DAC design by Carbon Engineering.
Regarding our PEM unit: The world’s most efficient and reliable Electrolyzer M4000 by Nel Hydrogen will be using for our design. Based on research the M4000 is the most feasible Electrolyzer for our design for large scale hydrogen production. One unit produces 4000 normal cubic meters per hour of hydrogen, which is equivalent to 359.52 kg/h of hydrogen. These specs along with other specs have been used to validate our design and ensure we meet all requirements for hydrogen production.
FEASIBILITY OF OUR DESIGN SOLUTION
As mentioned before, the liquid solvent DAC process presented here is inspired and closely following the research and pilot plant design by Carbon Engineering. As proven by their successful pilot plant in British Columbia, the technology itself is feasible with the downside of high startup cost. Economically, the methanol production may not meet the investment and capital cost required to build and utilize the CO2 generated by the DAC process. However, it is essential to compare this cost to the cost expected from mitigating climate change in the near future. Some of the reports expect Canada to spend around 25 to 176 billion dollars on reducing the damage done by climate change. Also, the effect it will have on people’s lives is unmeasurable. In that manner economic feasibility is not the biggest challenge that the DAC process has. With traditional energy sources such as natural gas emitting up to 620 gCO2/kWh in Alberta, DACI’s use of renewable energy (with emission factors of 10-80 gCO2/kWh) substantially reduces our emissions. This is the most important factor for the feasibility of our design, to ensure climate benefits, it is necessary to utilize low-carbon electricity.
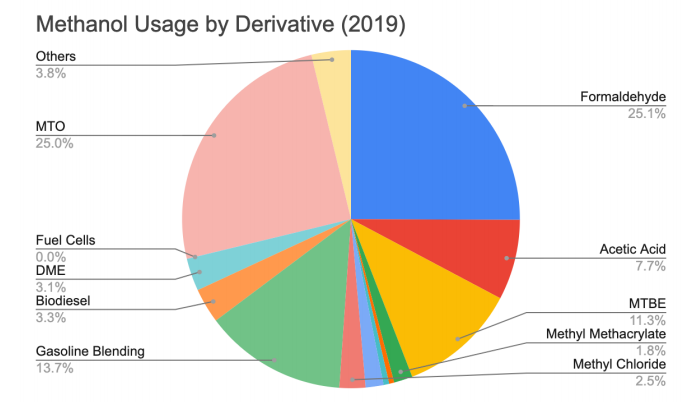
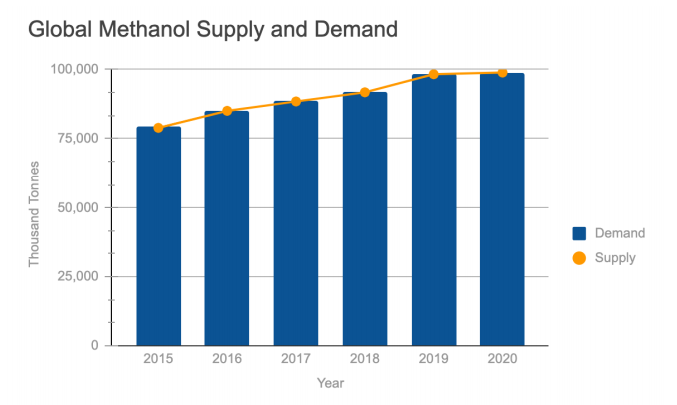
Based on our market analysis and economic assessment, this process could also profit from selling the excess carbon dioxide, oxygen and hydrogen it produces. Over the last several years, the idea of a “Methanol Economy” has emerged within the energy industry; it replaces petro-chemical based fuels and chemicals with methanol and methanol derivatives as a cost-competitive means in the global search for more sustainable fuel alternatives. Methanol is known for its versatility in producing various chemical derivatives and as a safe and clean energy source, methanol’s environmental advantages make it an attractive alternative in many industries. Major methanol derivatives produced in 2019 are shown below including the global methanol supply and demand from 2015-2020.
PLOT PLAN AND LAYOUT
The location chosen for our process was Medicine Hat, Alberta. All three processes, DAC, PEM, and Methanol Synthesis fit on roughly 2.6 km2 of land (1 mile2). This location was chosen for several reasons:
- Large sequestration opportunities
- Local industries
- Milder Southern Climate
- Wind Conditions
- Trans-Canada rail access
The plot plan of our process can be seen below:
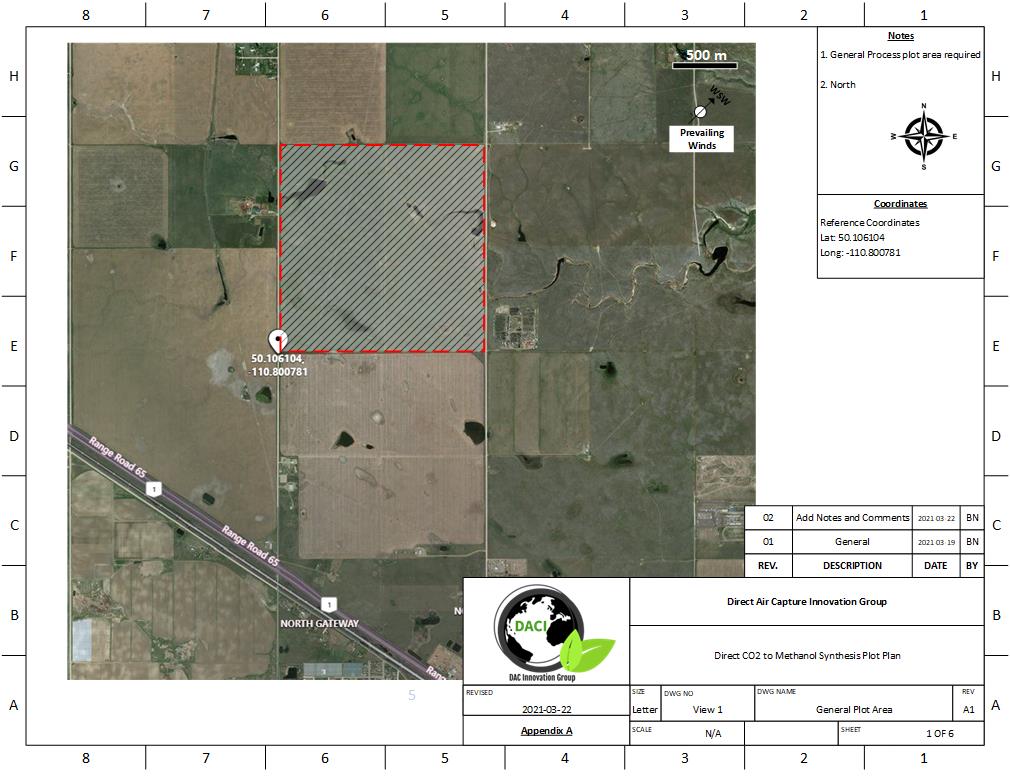
General Plot Area 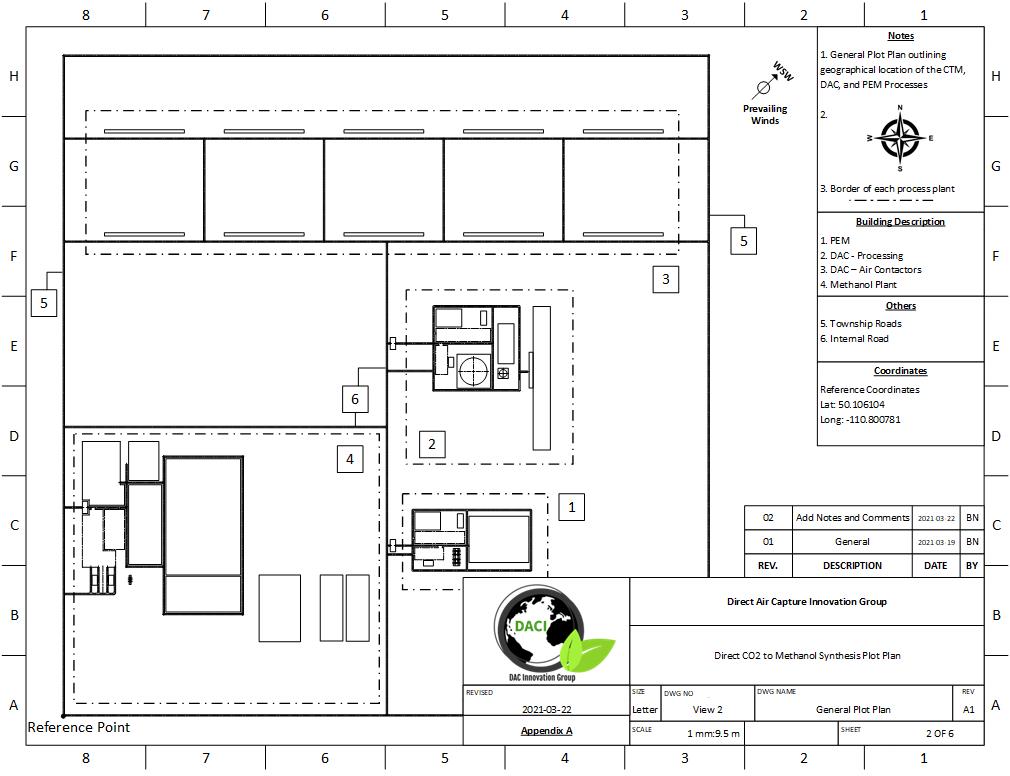
General Plot Plan 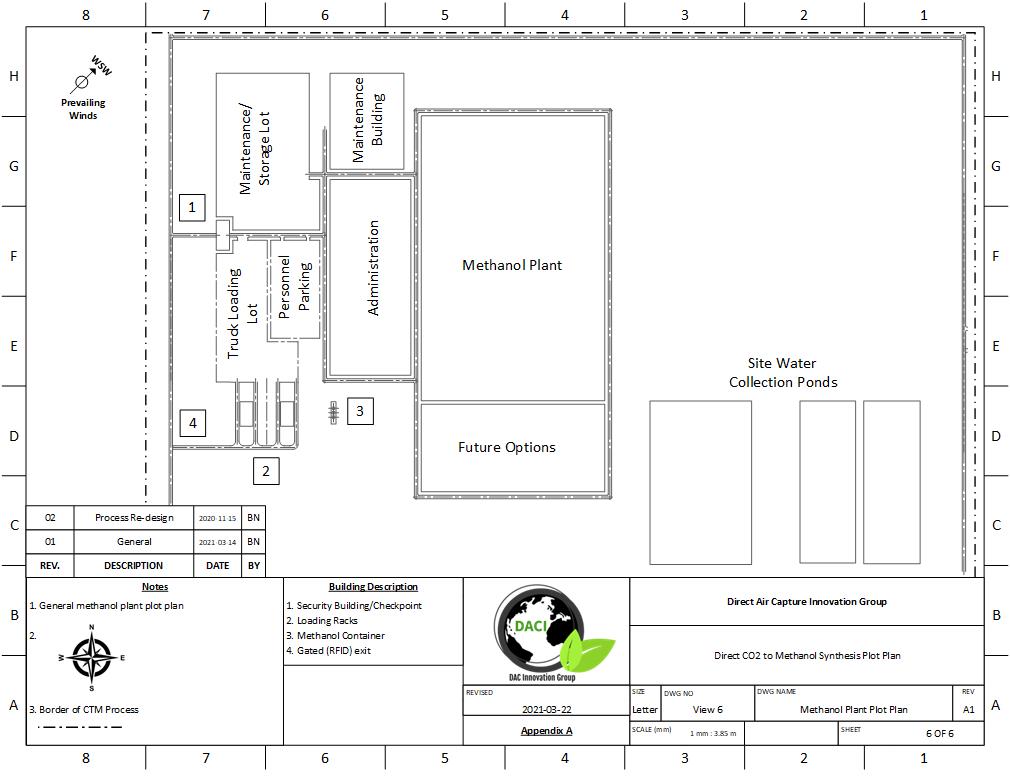
Methanol Plant Plot Plan 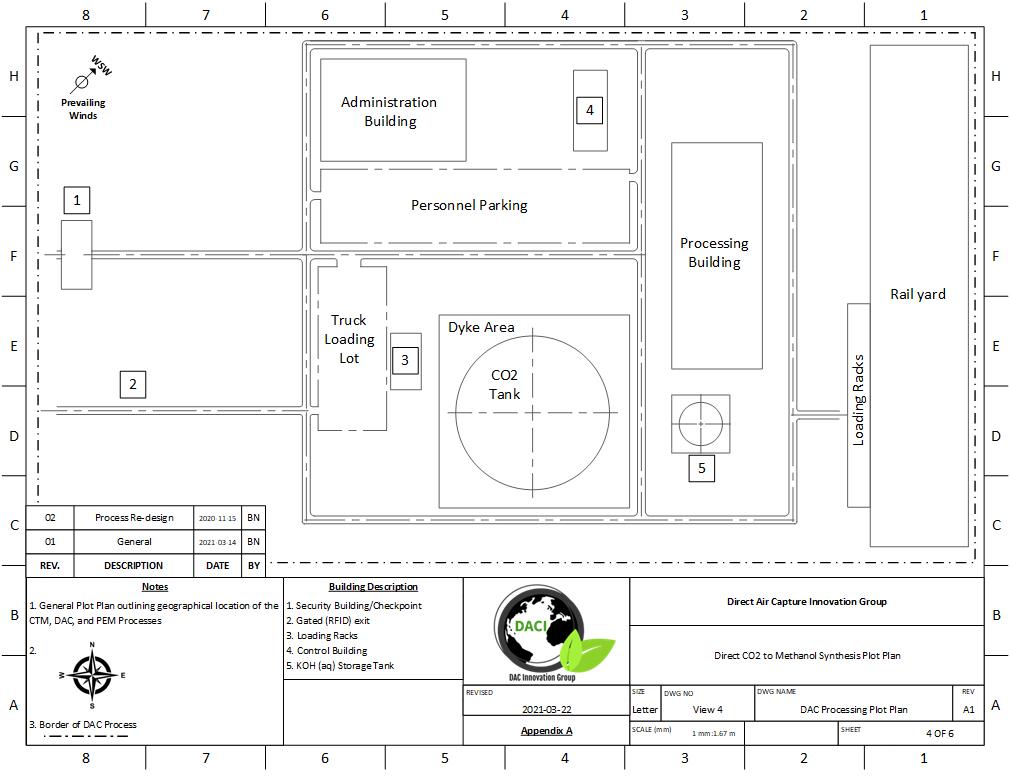
DAC Processing Plot Plan 
PEM Plot Plan
Partners and mentors
We want to give many thanks to our partners and mentors for their support.
Our supervisor professor Md Kibria and our teacher assistant Muflih Adnan guided us through the process with patience and great advice
and our course professor, Hector De la Hoz Siegler.
Our photo gallery

Conceptual image of the Direct Air Capture Unit called the Air Contactor
Source: Carbon Engineering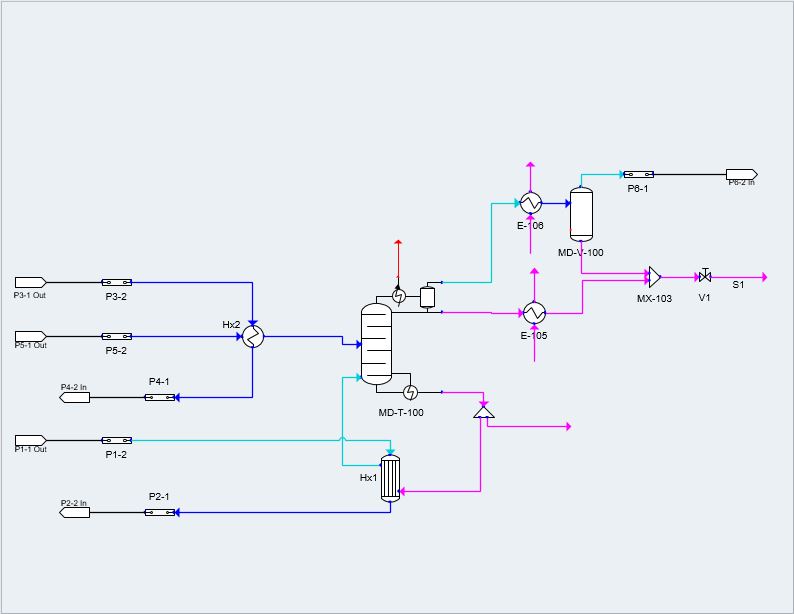
Symmetry Model 1 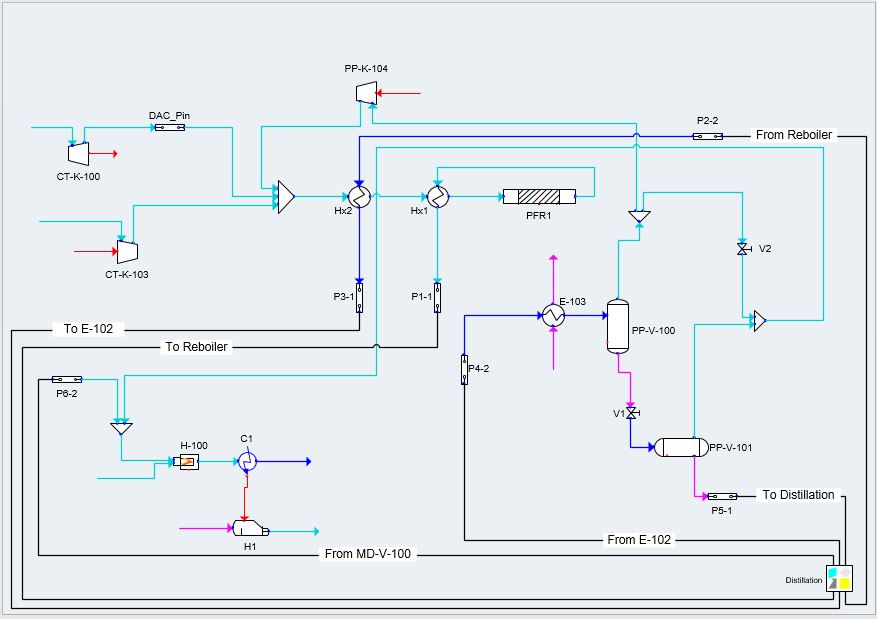
Symmetry Model 2 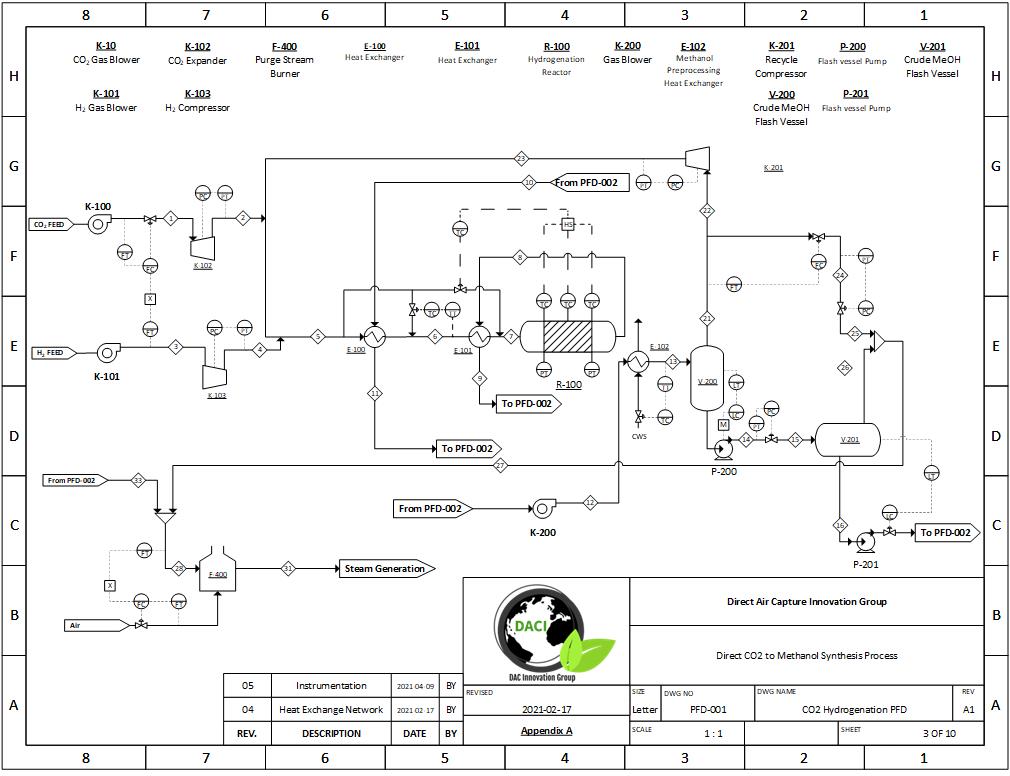
PFD Example 1 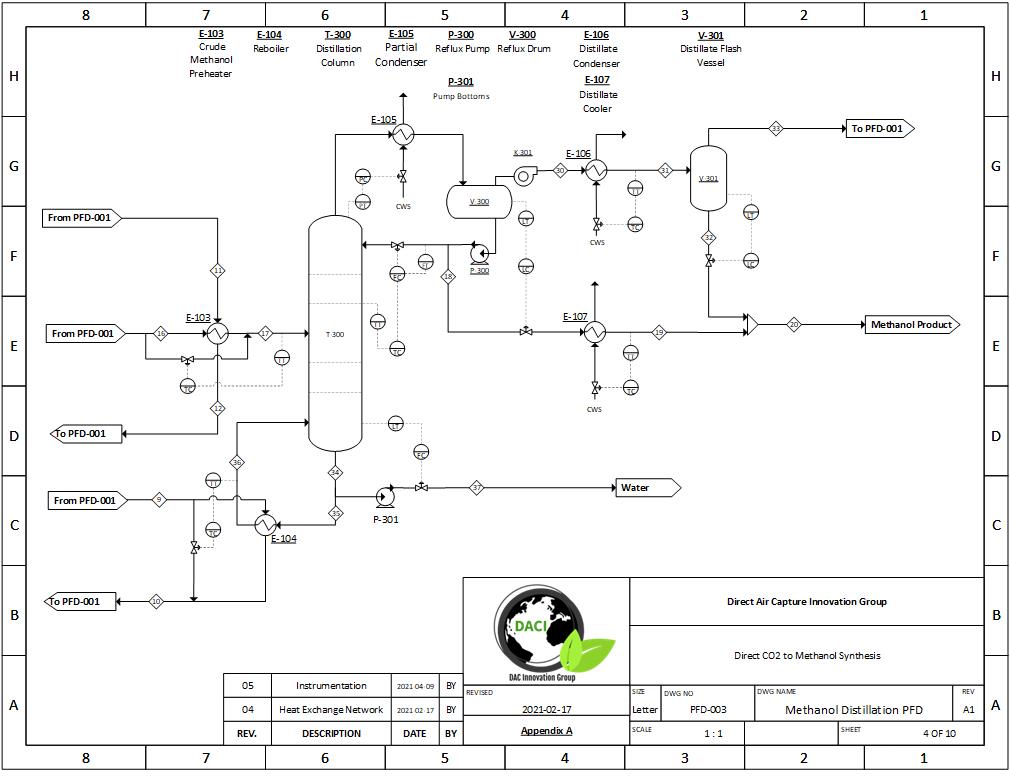
PFD Example 2