Project Category: Chemical
Presentation Chat Room
About our project

Creo Generations has developed an unconventional NTP reforming process to create an inherently safer gasoline with low life-cycle greenhouse gas (GHG) emissions to provide a sustainable gasoline source for transport in rural areas.
NTP reforming processes a straight-run naphtha feedstock to yield a higher-octane gasoline blending component dominated by clean-burning isoparaffins.
To supply activation energy at ambient pressures and low temperatures, an electrical current generates high-energy, low-heat ions, metastables, and free electrons inside the main reactive assembly. NTP particles have high kinetic energy, but negligible mass that keeps heat low. This process suppresses aromatic production while minimizing octane losses.
So what?
Creo Generations’ naphtha reforming design has 3 key advantages:
- Reduced hazards at ambient temperature and pressure
- Electrical power can be supplied by green energy
- Product has low levels of toxic aromatics, reducing end use particulate emissions
Meet our team members





Why gasoline?
TRANSPORTATION EMISSIONS
Transportation is a major source of pollution, emitting 14% of global greenhouse gas (GHG) emissions directly. A wide range of solutions have been investigated or implemented to address these impacts:
- Accessible mass transit infrastructure and transit electrification
- Pedestrian– and bike-friendly traffic engineering
- Carbon-neutral biofuel development (Clean Fuel Standard)
- Electrical vehicle manufacturing and infrastructure development
- Hydrogen chemical energy development

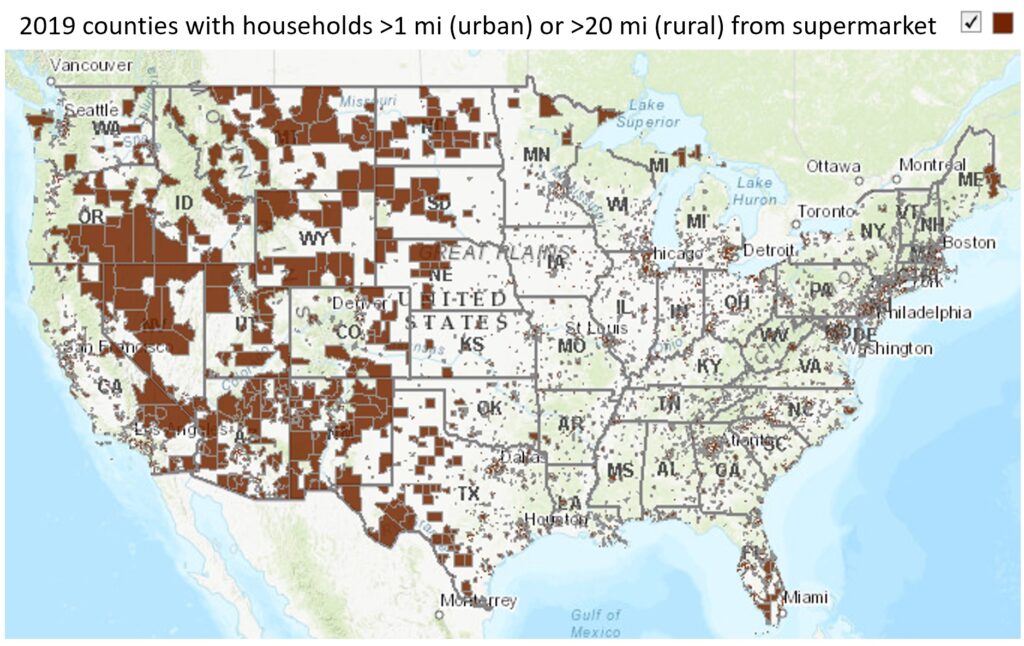
LIMITED ALTERNATIVES
Many of these options rely on strong infrastructure or accessible local amenities. Even in highly developed countries, many people are unable to access necessities by foot or bike and do not have access to mass transit services.
Rural transportation will remain gasoline-dependent for power in spite of green energy development. Low-impact gasoline with a more sustainable production cycle and less deadly exhaust will help slow the effects of this continued use.
LOW-IMPACT GASOLINE
The European Union and California have taken the first step in mandating an inherently safer gasoline by capping aromatic content at 30%. Creo Generations anticipates that these regulations may become more widespread, increasing the need for low-aromatic gasoline.
Aromatics are toxic hydrocarbons that improve gasoline performance. When burned, they emit high levels of smog, causing respiratory illness and premature death. However, removing aromatics has one challenging side effect: it lowers the performance quality (octane number) of gasoline.

What is naphtha reforming?
Gasoline is a light fuel blend with an octane number of 86 or above in North American markets. A high octane number (86 or above) signals that the fuel is unlikely to cause engine damage from premature combustion. Typical gasoline contains:
- Straight-run naphtha (C6-12 naphthenes)
- High-octane additives
- Naphtha reformate (C6-12 aromatics)
- Butane (C4 gas, dissolved)
- Alkylate (C7-9 isoparaffins)
- Ethanol (alcohol)
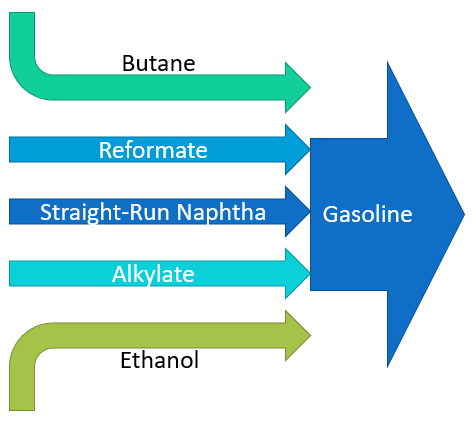
Conventional naphtha reforming converts naphthane molecules to aromatics. Creo Generations has designed a reforming process that targets isoparaffins over aromatics, similar to alkylate.
The key difference between alkylation and reforming is the feedstock: alkylation combines light gases into larger, branched paraffins, while reforming takes a naphtha feed and alters the structure rather than the size of the molecules.
Details about our design
INNOVATING AN INDUSTRIAL MAINSTAY
Naphtha reforming is a widely-used process with one goal: convert naphtha to aromatics. Targeting isoparaffins in the product required a number of adjustments to compensate for this initial change.
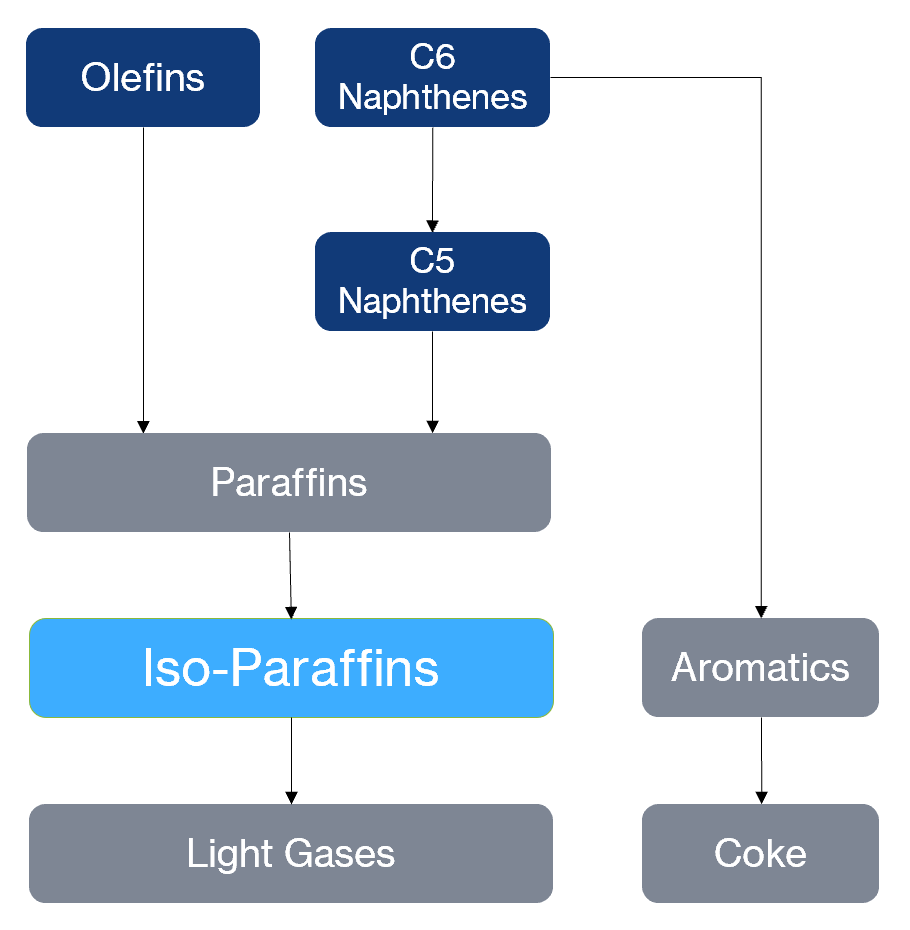
The conventional reforming pathway is endothermic, dehydrogenating naphthenes and dehydrocyclizing paraffins and olefins over a platinum-based catalyst to yield aromatic molecules. This process takes place at 500°C and 3-35 atm of pressure, requiring several runs through a fossil fuel furnace to reheat the naphtha during these reactions. The hydrogen co-product is recirculated to the reactor to prevent coking.
Non-thermal plasma (NTP) reforming chooses an exothermic pathway instead, where naphthenes and olefins are saturated with hydrogen to form paraffins, which isomerize to isoparaffins (branched paraffins). The process takes place at ambient pressure and a low temperature of 50°C, just above the mixture dew point. A methane (CH4) co-feed is added to compensate for the lost hydrogen, since each molecule has a high hydrogen:carbon ratio.
To break apart the molecules in the feed, more energy needs to be added in the form of non-thermal plasma, a mix of free electrons, metastables, and ions with high kinetic temperatures and low mass. These individually have sufficient energy to trigger reactions, but not enough to heat up the reactor.
Argon carrier gas has to be added to the reactor to make sure the plasma travels freely in the reactor, increasing conversion. The argon:hydrocarbon ratio in the reactor is approximately 4:1.
The main challenge Creo Generations encountered was achieving an optimal conversion midpoint. Experimental data indicated that paraffins are the intermediate step between the naphtha feed and the desired isoparaffin product. However, it was also observed that isoparaffins cracked to form light gases instead of paraffin. At high feed conversion, isoparaffins are lost as gas. At low feed conversion, low-octane paraffins dominate the product.
PLANT UNITS
NATURAL GAS PROCESSING
Raw U.S. natural gas can contain up to 40% nitrogen (N2) gas, an inert substance that could yield undesirable byproducts in the NTP reactor such as toxic ammonia gas (NH3). A Pressure Swing Adsorption (PSA) unit can isolate a 97% pure methane (CH4) stream from natural gas for use in the reactor.
The PSA unit includes 2 columns packed with carbon molecular sieve (CMS) adsorbent. 1 column adsorbs N2 from the raw gas stream while 1 column desorbs N2 into a 97% pure methane purge. The raw gas feed diverts to the regenerated column if the exit stream purity drops.
VALIDATION
TThe PSA Unit was designed based on an assumed breakthrough time of 600 s, derived from literature. A mass balance on the solute (N2) was then used to find the length of the PSA unit with the diameter assumed to be 25% of the length according to heuristics in literature.
The Klinkenberg equation was then applied to the calculated length and literature values for N2, CH4, and CMS properties to check the assumed breakthrough time. The iterations were done using Microsoft Excel until the calculated and assumed times were closest.
NON-THERMAL PLASMA (NTP) REFORMING
The core of the process is reforming, converting naphthenes, paraffins, and olefins of 6-12 carbons (C6-12) into branched isoparaffins by generating non-thermal plasma.
Each NTP reactor is a narrow glass tube with a coaxial wire inner electrode and a coil outer electrode, both stainless steel. Reactors packed with Ga/mix-ZSM-5 catalyst are connected in series to make up the total NTP reactor assembly volume.
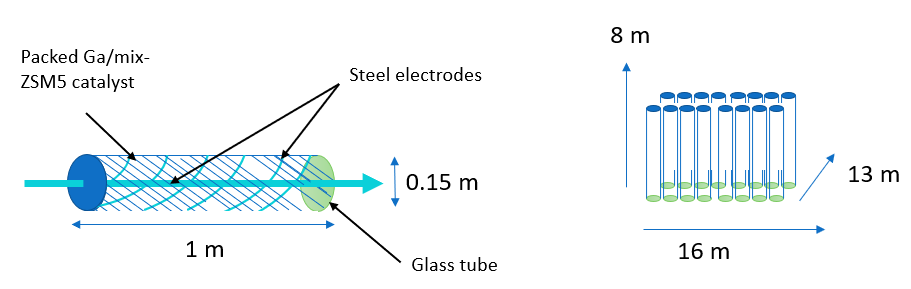
Vaporized naphtha, natural gas, and argon enter the assembly at 1.5 atm as electrical current is passed through the inner electrode. This current generates non-thermal plasma metastables, ions, and electrons to trigger reforming reactions.
The high proportion of inert gas means that plasma does not immediately react when generated, travelling through the reactor instead of creating a small reaction zone near the electrode in the centre of the reactor.
VALIDATING AN NTP REACTOR
Naphtha is a hydrocarbon mixture that can contain over 100 distinct compounds in varying proportions. Creo Generations generalized this composition into 45 component lumps after an in-depth review of literature naphtha reforming models. A non-equilibrium kinetic model was built in MATLAB to reflect the non-equilibrium thermodynamics of NTP. After building a basic model, changes were implemented to account for pressure drop, reaction heats, and catalyst deactivation from coking.
The model results were validated by comparison with experimental NTP reforming data from a literature source. Final model output accuracy was +/- 30% when compared with the experimental product for the same feed.
FUEL DESULPHURIZATION
Alberta crude oil is high in sulphur, containing up to 4% sulphur by weight. When burned, high-sulphur fuel produces sulphur oxide gases (SOx) that contribute significantly to ocean acidification, erosion, and ecosystem poisoning.
In conventional reforming, the naphtha feed is desulphurized to prevent catalyst poisoning in the reactor. Creo Generations has used the HDS unit as the last step to take advantage of 60% desulphurization in the reactor, cutting hydrogen (H2) costs.
VALIDATION
The HDS unit was simulated in Symmetry software. The material vapour and liquid flows resulting from the simulation were used in sizing calculations using textbook heuristics.
HDS is a common industrial process used in most oil refining facilities. After calculation, sizing results were validated by comparison with industrial HDS units sizing from literature.
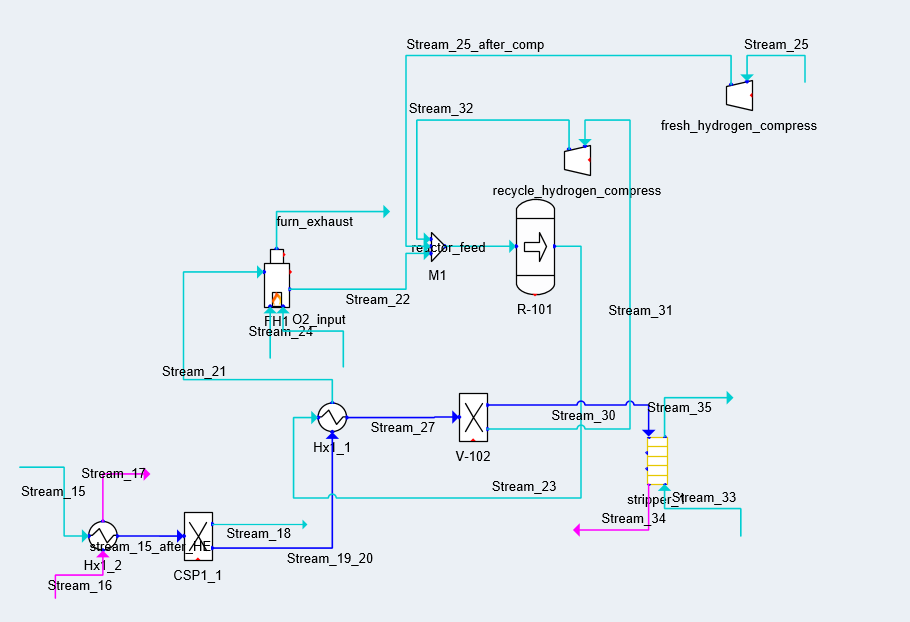
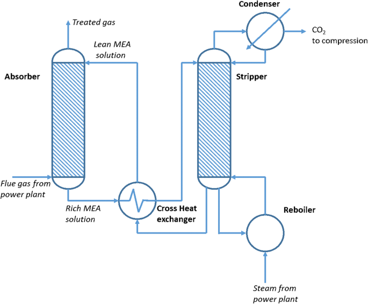
WASTE GAS TREATMENT
Acid gas (H2S) is a toxic substance to be isolated and treated to reduce risks to plant workers and surrounding communities. Creo Generations has designed an amine contactor that will remove acid gas from the reactor recycle stream and divert it to existing sulphur recovery facilities at Cherry Point Refinery.
The solid sulphur obtained from the refinery desulphurization systems can be sold for fertilizer production and other industrial processes.
20% of the recycle stream is diverted to the refinery furnace for combustion to avoid C2-5 buildup in the recycle stream.
VALIDATION
The amine unit was simulated using Symmetry software. Amine contacting is often paired with HDS in industrial processes to capture the acid gas in the HDS reactor effluent.
The calculated amine unit sizing based on final simulation results was validated by comparing with literature sizing of typical gas sweetening systems.
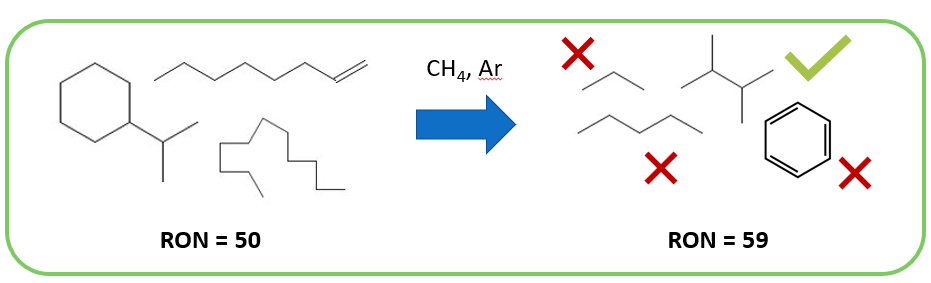
IS IT EFFECTIVE?
Creo Generations aims to convert straight-run naphtha into a high-octane mixture suitable for gasoline with minimal aromatic content. The design presented takes a 30 mol% isoparaffin feed and yields a 50 mol% isoparaffin product, increasing octane from 50 to 60. The final product requires significant blending with alkylate and ethanol before sale. To reach an octane number of 86, the final mixture must be 30 vol% NTP reformate, 10 vol% ethanol, and 60 vol% alkylate.
The blended gasoline product is 5 vol% aromatic, compared to 15-25 vol% aromatics in existing low-smog gasoline. This shows that NTP reforming can yield a low-aromatic gasoline with reduced hazards and the flexibility to use renewable power.
However, the tradeoffs between cracking, coking, aromatic levels, and octane number could not be reconciled under the current model to deliver large quantities of high-octane gasoline. Instead of acting a high-octane additive, NTP reformate is a substitute for straight-run naphtha with low aromatic content.
ECONOMIC FEASIBILITY
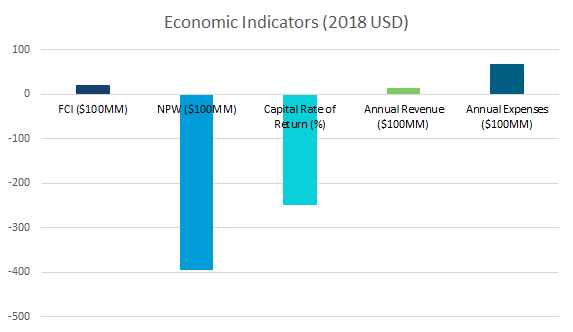
CNR reforming is a potent high-octane additive, used in small quantities to boost fuel performance. Rather than acting as a substitute for conventional reformate, NTP reformate is a substitute for straight-run naphtha that requires blending to reach the minimum octane value.
Because of the novel NTP technology used, capital costs for NTP reforming are significantly higher than CNR reforming while the product is lower-value. Factoring in the cost of additives to blend NTP reformate to a market quality, the proposed addition will not be profitable in the forseeable future with a net present worth of –$39B in 2018 USD.
The earnings before taxes, depreciation, and interest (EBIDTA) for the project are -$5.5B yearly.
Partners
Creo Generations is grateful for the support, advice, and insight of project supervisor Dr. Hua Song throughout the design process. We would like to extend a special thanks to PhD candidate Shijun Meng for supplying NTP reforming data and interpretation of this novel technology and to Mr. Rein Saar for his invaluable experience.
This project would also not be possible without the technical expertise and patience offered by Dr. Hector de la Hoz Siegler and Dr. Michael Foley.
Plans and Diagrams
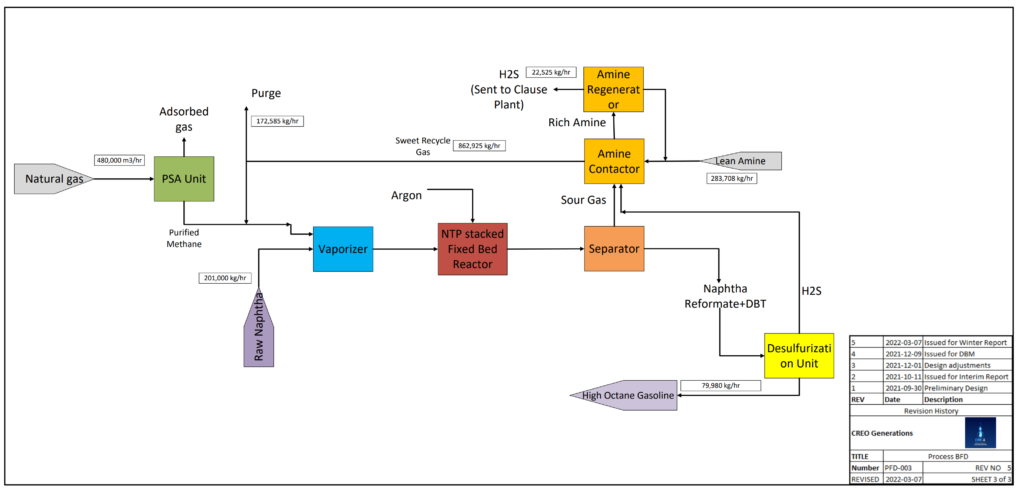
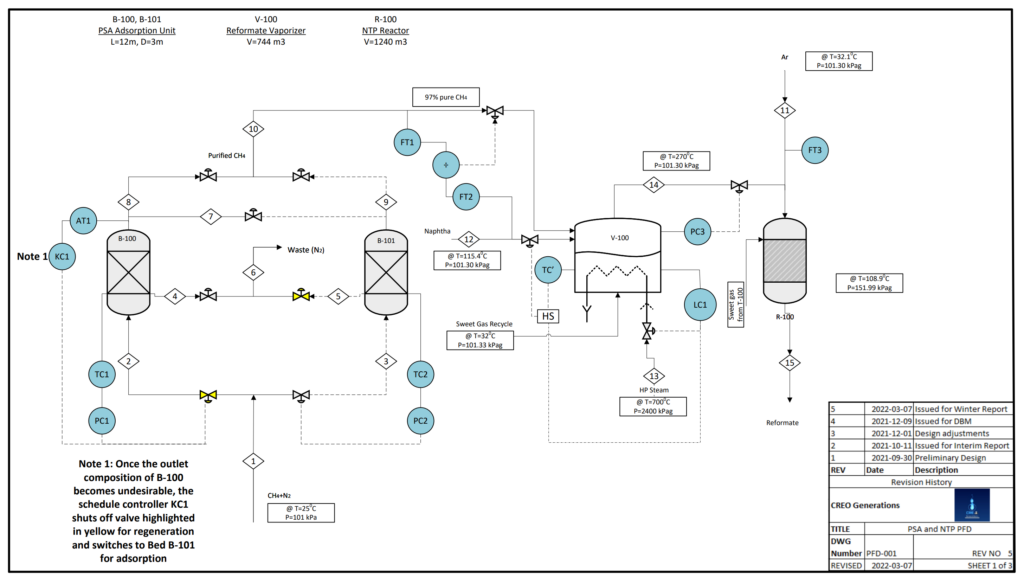
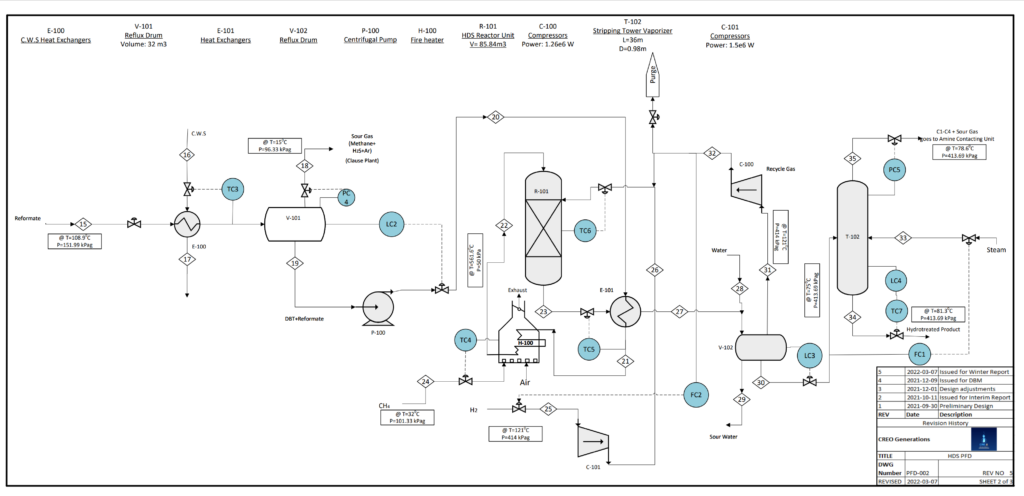
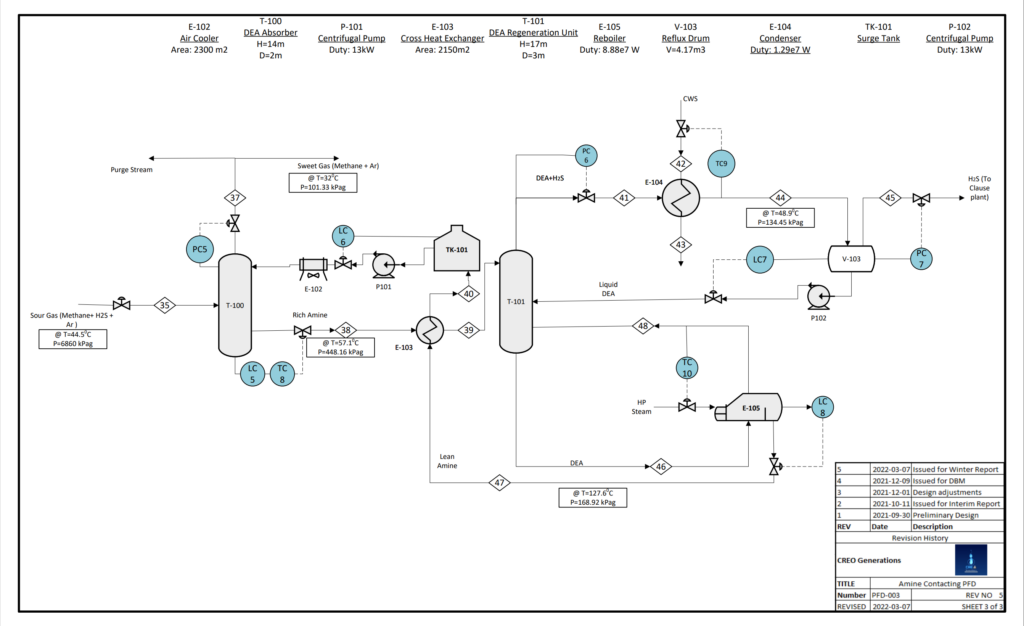
Image Sources
Figure 1:
Newman, P. How to build light rail in our cities without emptying the public purse, The Conversation. https://theconversation.com/how-to-build-light-rail-in-our-cities-without-emptying-the-public-purse-39255
Nicholas_T. Monoculture. https://www.flickr.com/photos/nicholas_t/4719020153/
Bexim. Hydrogen Fuel Station Sign. https://commons.wikimedia.org/wiki/File:Hydrogen_Fuel_Station_Sign.jpg
Figure 2: Food Access Research Atlas. U.S. Department of Agriculture. https://www.ers.usda.gov/data-products/food-access-research-atlas/go-to-the-atlas/
Figure 3: Tomskyhaha. Fanhe Town 10 Day Interval Contrast. https://commons.wikimedia.org/wiki/File:Fanhe_Town_10_day_interval_contrast.png
Figure 9: Adamu, A.; Russo-Abegao, F.; Boodhoo, K. Process Intensification Technologies for CO2 Capture and Converstion – A Review. BMC Chemical Engineering, 2020, 2, https://doi.org/10.1186/s42480-019-0026-4.
