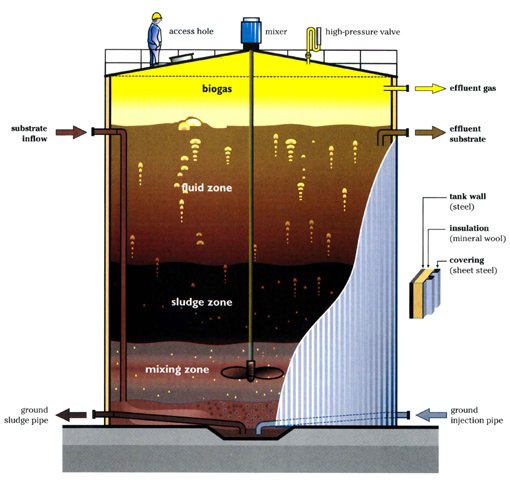
About Canathol
Canathol provides a high quality and extremely pure ethanol product in addition to the production of biogas to power our facility. Our ethanol is of 99.95% purity and can be utilized in multiple industries including pharmaceutical, cosmetics, automobile, household cleaners, toiletries, and many more. Our process takes cannabis waste and converts it into a value-added product, contributing to a healthier environment through multiple avenues.
The main units involved in our facility includes steam explosion & acid hydrolysis, enzymatic hydrolysis, ethanol fermentation, ethanol purification, and anaerobic digestion. This is a complex process requiring multiple stages of optimization and integration. As this process involves various biological reactions, certain variables such as the lifetime of bacteria and enzymes in our process became the focus of our research when implementing a suitable reaction and determining the best operating conditions. Canathol welcomes you to explore our process in more detail below. The Canathol team will be available to answer any further questions and inquiries that you may have in our virtual booth.

Our Journey
To learn more about Canathol’s journey, please click on the video link below.
High-level Overview of Canathol’s Design
HOW OUR DESIGN ADDRESSES PRACTICAL ISSUES
With climate change becoming an increasingly important agenda item for countries around the globe, diversifying away from non-renewable fossil fuels will be paramount for the remainder of the century. Canathol provides solutions to this end, with production capability of bioethanol and biogas derived from cannabis waste. Our process solves two issues by taking care of waste and turning it into value added products.
Locating Canathol’s plant in Alberta also provides diversification of the economy away from a traditionally hydrocarbon-based province turning Alberta into a leader in sustainably manufactured fuels.
In the future, Canathol’s process may be adapted to process additional agriculture feedstocks in order to scale up the production of bioethanol and biogas as per commodity demand.
As the need for more sustainable sources of energy and important chemical compounds increases, the value of using a waste product as a feedstock becomes apparent. Canathol’s process has never been implemented in Alberta, and with a growing cannabis market our process is at the forefront of utilizing the wastes and turning them into value added fuels.
In addition to producing bioethanol, Canathol’s process also produces biogas in order to power the plant and make the overall process more energy efficient. This co-production process further differentiates us from our competition and provides a more self-sufficient process as by utilizing biogas to generate electricity onsite for utility needs.
WHAT MAKES OUR DESIGN INNOVATIVE
WHAT MAKES OUR DESIGN SOLUTION EFFECTIVE
Bioethanol is produced via microbial fermentation using carbohydrates from sugar and starch bearing plants. Common feedstocks include corn, barley, wheat, sugarcane and sweet sorghum. Corn is by far the most popular feedstock for industrial bioethanol production in the Unites States and Brazil, wheat and sugar sorghum in Europe.
Using the carbohydrates mentioned above creates two major consequences:
- Large amount of arable land is required to grow the crops for production affecting biodiversity and natural habitats existing in the area.
- Usage of corn, wheat and other carbohydrates for bioethanol production indicates a lower supply of these products for the food industry decreasing overall consumption.
Our design solution to produce bioethanol using hemp hurds over corn or other sugars is effective in that we are utilizing a waste product that would otherwise get dumped to landfills or incinerated on site. Additionally, hemp hurds cannot be used to manufacture food products for human consumption like common agricultural feedstocks can.
All research projects begin with a thorough and detailed background review using available literature and consultations with subject matter experts (SME) in the relevant industry. The literature review period occurred in the earlier stages of the project and lasted several months as the team gathered information on the inner workings and process necessities of a biofuel facility. We looked at papers from topics on bio-concrete to plastics before selecting bioethanol and biogas as the best combination of products for the market and available resources in Alberta.
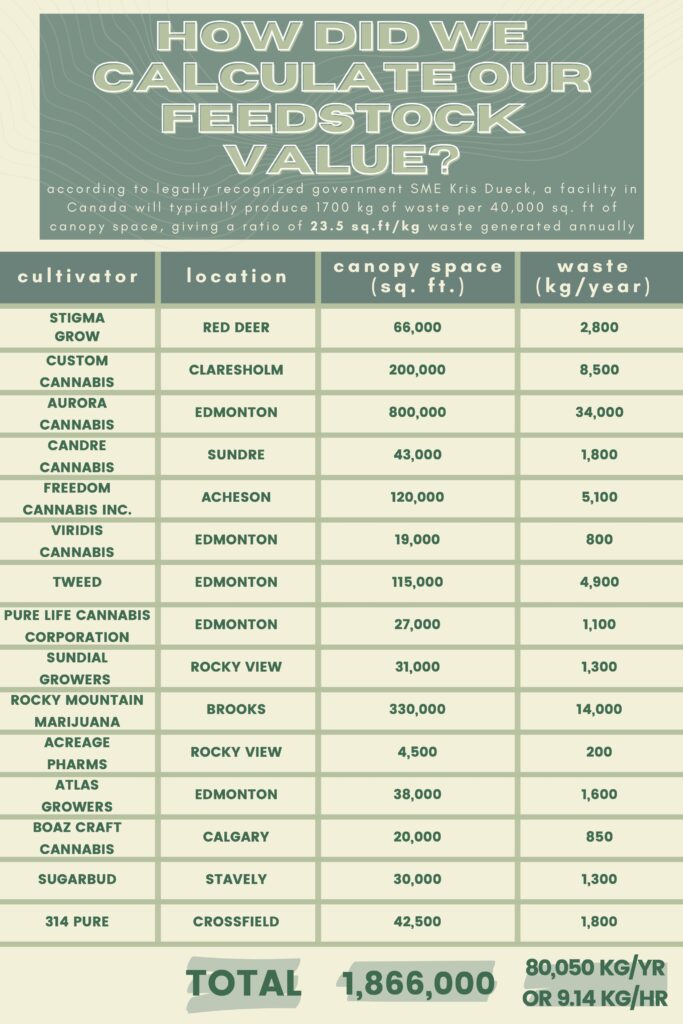
Through our client’s connections, we were able to have interesting and thought-engaging conversations with SMEs Marek and Kris Dueck. These individuals are experts in the Cannabis industry and consultants to multiple cultivators in Canada, providing their knowledge to our team on the ideal operating conditions and limitations of the design. Our feed numbers for the hemp hurds in the facility are guided by their expertise on average waste production rates.
Our team used multiple simulation software tools such as Aspen HYSYS, Matlab and VMG Symmetry to model the various biological reactions and separation processes involved in the project. We validated these simulations by using the starting feed numbers from various published journals with the same model as our units and conducted an assessment on whether we obtained similar end results to the papers. If we observed significant differences in the results, we would adjust specific parameters in the model to ensure higher accuracy and optimal performance. After completing all of those simulation runs, our model validation studies indicate a low percent error of <5% for all major pieces of equipment, indicating that our software simulations are reliable and accurate.
HOW WE VALIDATED OUR DESIGN SOLUTION
FEASIBILITY OF OUR DESIGN SOLUTION
Canathol has conducted a thorough economic evaluation on the plant’s costs and projected revenues over 25 years of operation and has concluded that the project is economically unfeasible with the limited amount of feedstock that is available for use. With an hourly feedstock flow rate of 9.1 kg/hr, the calculations for which are shown below in ‘Evaluation of Feedstock’, the facility will produce approximately 3.8 kg/hr of bioethanol. This is equivalent to around an annual production rate of 32,000 L/year, which would generate a total revenue of $35,000 USD annually. The breakeven selling point for ethanol must be 760 USD/gal or 200 USD/L for our facility to produce enough revenue to operate, which is in line with what cannabis industry experts, Kris Dueck and Marek Hejduk, predict for a project such as ours to be feasible. In the future, this project may become successful if government incentives are provided to the bioenergy industry, as it would reduce in-house costs to purchase equipment and land, thereby greatly minimizing the costs of operation.
From the beginning, both Canathol and the client understood that this project had the real possibility of failing economically. However, the purpose of this project was to demonstrate that cannabis waste collected in Alberta could be upgraded into valuable products that diversify our economy and energy market. The project was still a major success, both by our standards and our client’s, as this is proof-of-concept that a process to upgrade cannabis waste into bioethanol and biogas is technologically feasible. Canathol hopes that, one day in the near future, the provincial government provides fiscal incentives to both the cannabis industry and the bioenergy industry. Only with the support of the government are we able to showcase the full positive economic and environmental potential on the current Albertan market of a bioenergy-focused project such as ours.
Canathol’s Detailed Design
The following section provides a more in-depth explanation of the process of converting cannabis waste into bioethanol and biogas.
EVALUATION OF FEEDSTOCK
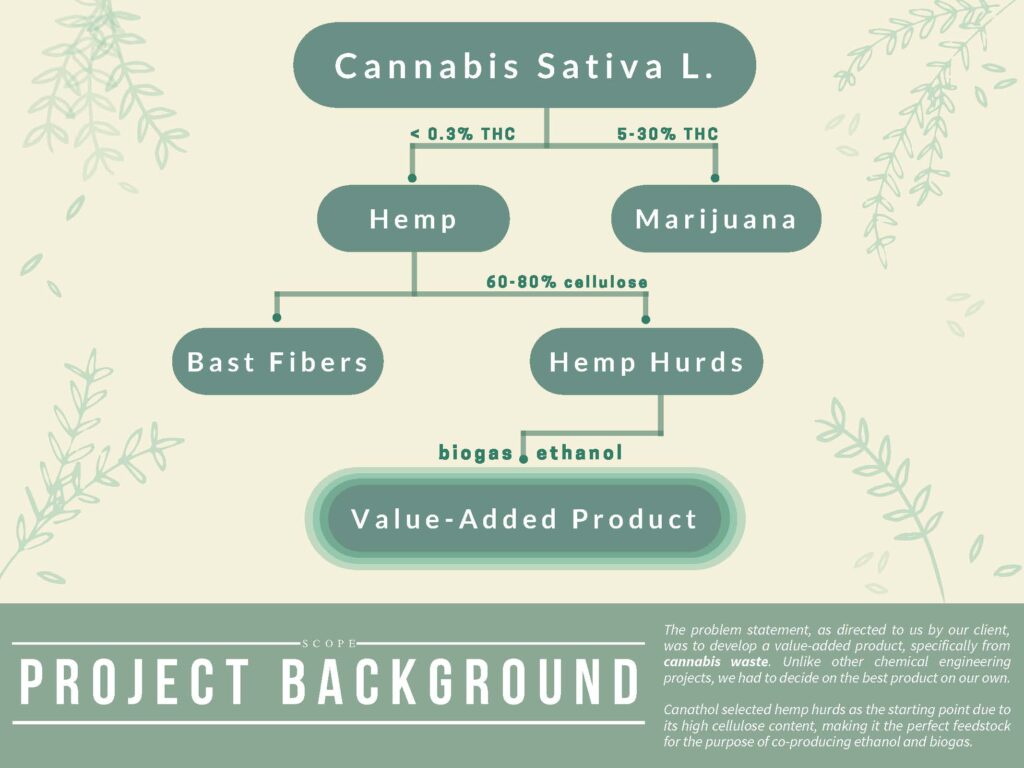
Cannabis waste is typically composed of two different materials, (i) bast fibers, and (ii) hemp hurds. Canathol has selected hemp hurds as the feedstock for our process since further treatment to reduce the THC content is not required as it would be with marijuana waste, the biomass is high in cellulose content, and the alternative bast fibers are an established feedstock in the paper and textile industry, signifying that there is less competition present in the hemp hurds industry. Take a look at Canathol’s calculations below for details on how our feedstock value was determined, cannabis industry expert Kris Dueck was consulted for these values.
PROCESS DETAILS
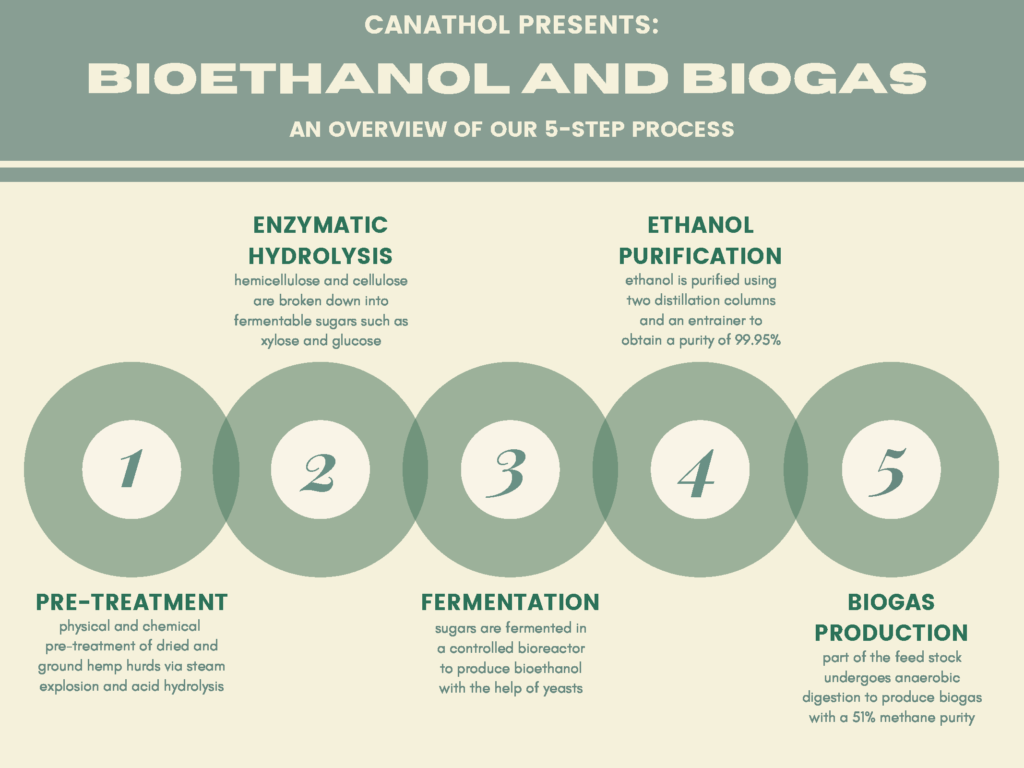
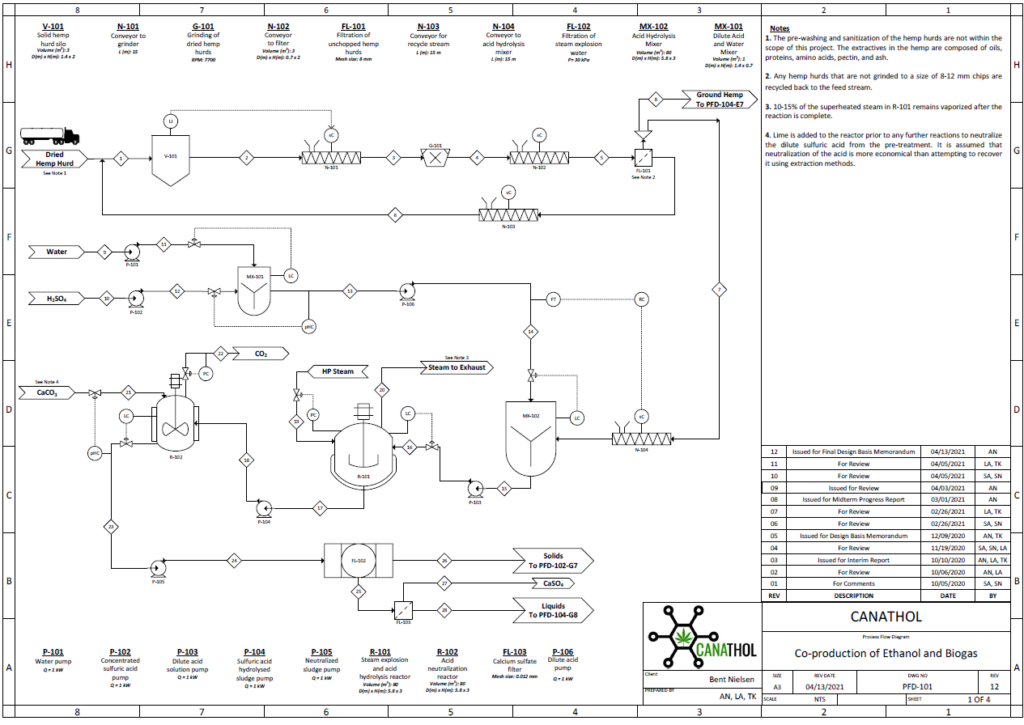
Steam Explosion and Acid Hydrolysis
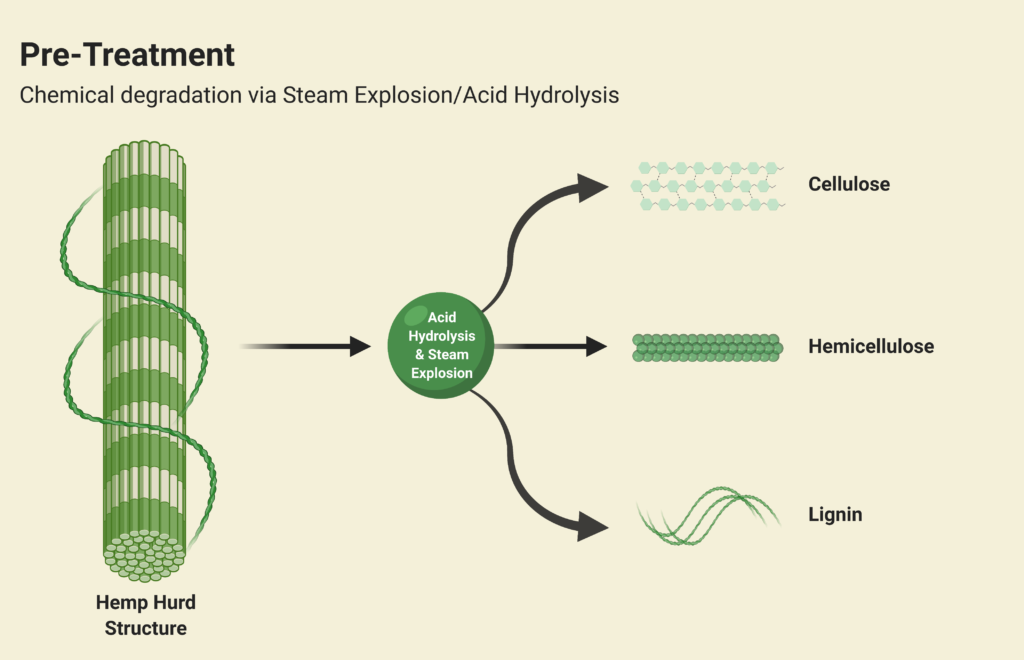
The physical and chemical pre-treatment is Step 1 of Canathol’s process. It takes place in a continuous stirred tank reactor (CSTR, R-101) at a temperature of 200˚C and pressure of 10 atm for the steam explosion of the hemp hurd slurry in the reactor during a 20-minute residence time. The main purpose of the pre-treatment is to separate the tightly wound lignin, hemicellulose and cellulose apart from each other in the hemp hurd. Some of the cellulose and hemicellulose also converts into glucose and xylose in this initial step. Approximately 8 kg/h of feed is being fed into R-101 with 37 kg/h of high-pressure (HP) steam being injected as well. The residence time of the acid hydrolysis was optimized to be 20 minutes via AspenPlus simulations.
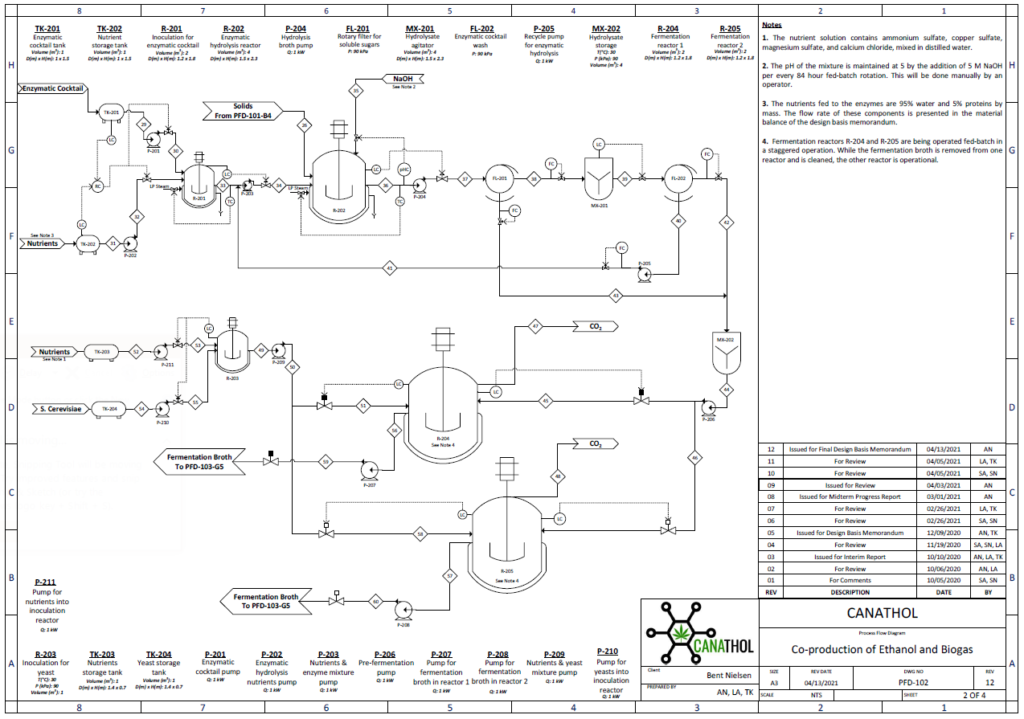
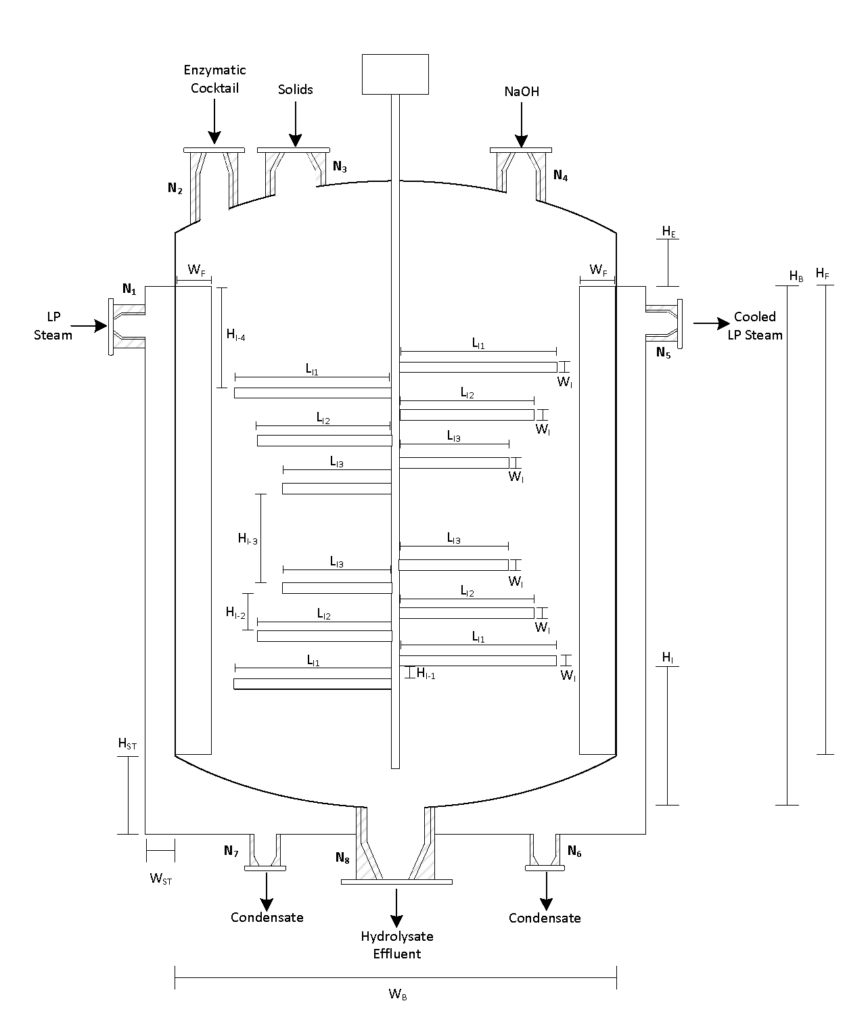
Enzymatic Hydrolysis
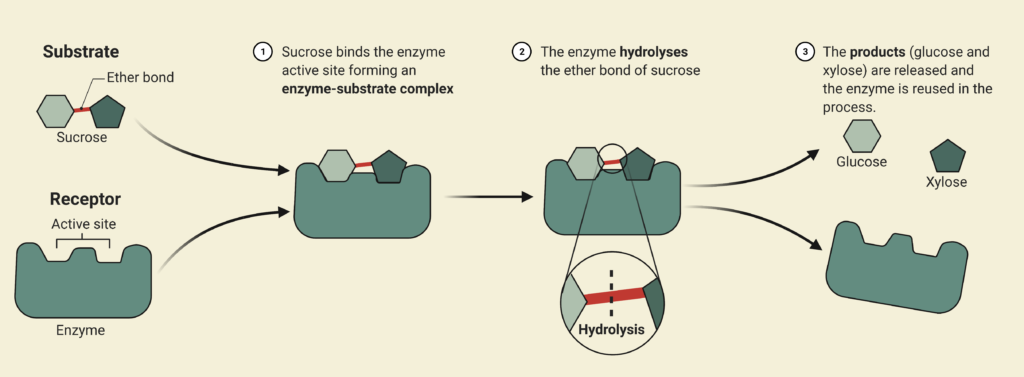
During the enzymatic hydrolysis or saccharification of lignocellulosic biomass, glucose and xylose are formed by the conversion of cellulose and hemicellulose catalyzed with the aid of enzymes. The objective of the reaction is to liberate any hemicellulose and cellulose bound by lignin and convert it into some variation of glucose so that yeasts can ferment them into bioethanol in Step 3. The processes are facilitated by the addition of an enzymatic cocktail which is composed of a commercially supplied mixture of hemicellulases and cellulases at optimal concentrations. These enzymes are expensive to re-purchase so Canathol has implemented a liquid recycled system using a combination of rotary filters allowing for 87% recycling and further reducing operating costs. After optimization of design parameters using the Michaelis-Menten kinetic model via MATLAB, the prime temperature for the reaction is determined to be 50oC for a residence time of 84 hours, achieving a conversion of 59% and a selectivity of glucose production over cellobiose of 38.
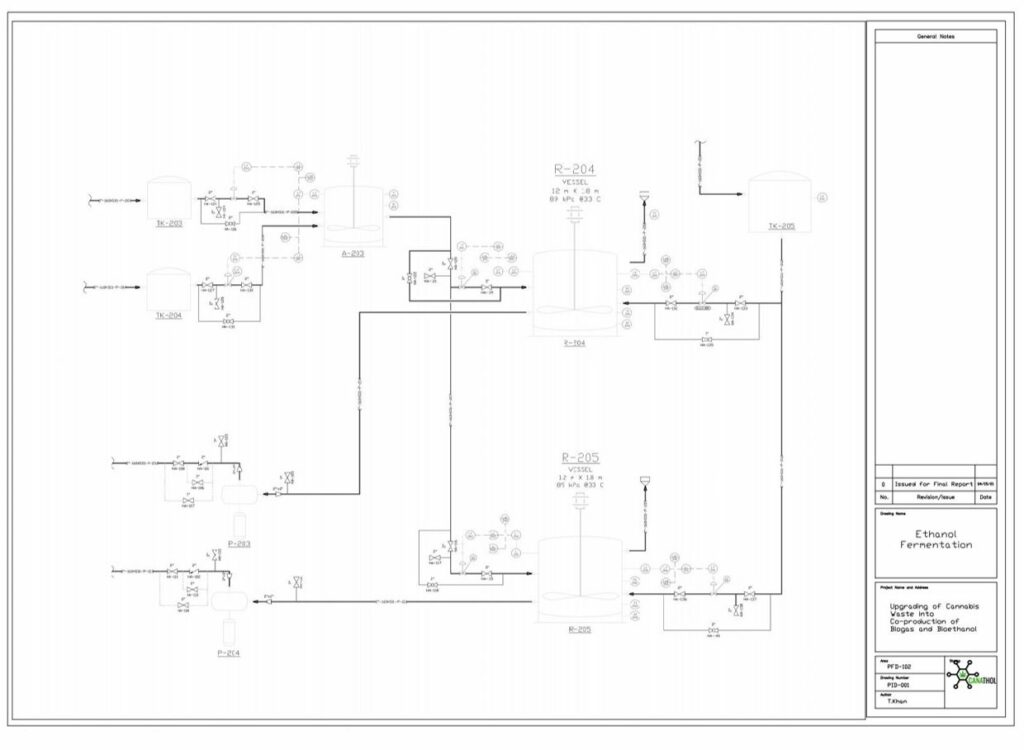
Fermentation
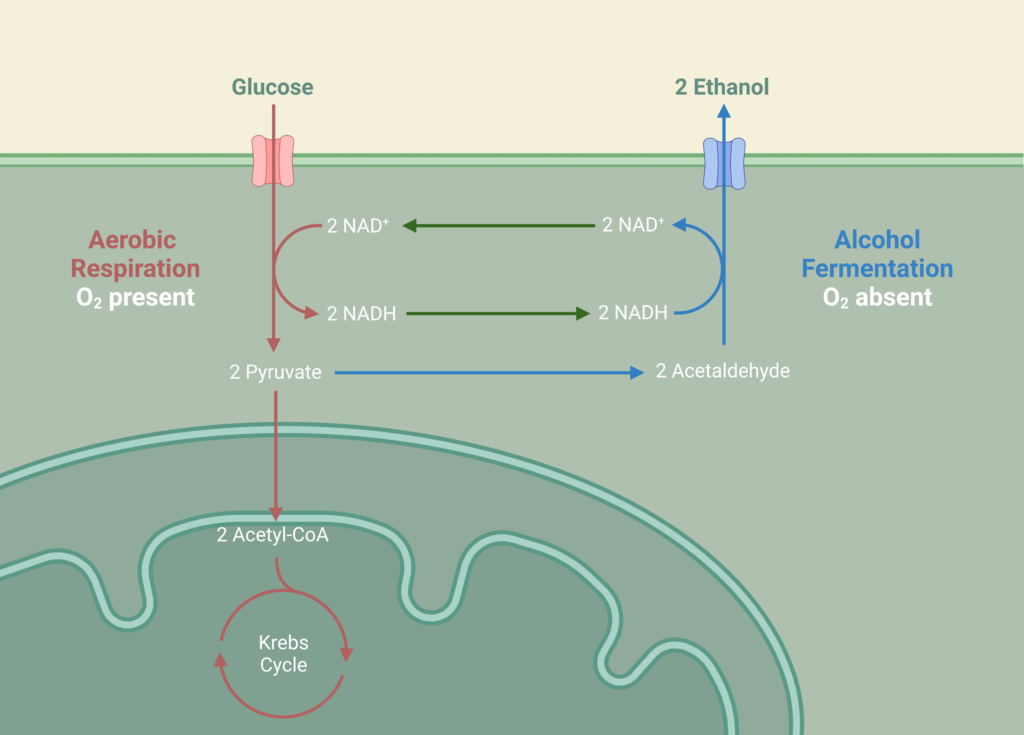
Ethanol fermentation occurs in two parallel CSTRs using the hydrolysate effluent from the hydrolysis reactor as feed which is composed of glucose, xylose, glucan, and lignin at a temperature of 33°C and pressure of 1 atm. Fermentation of the sugars in the reactor is carried out by the yeast species, Saccharomyces Cerevisiae IPE005. This particular yeast was selected as it has high conversion rates for the mixture of glucose and xylose into ethanol while being relatively unaffected by furfural at low concentrations, in comparison to other yeasts. The yeast is first exponentially grown in an inoculation tank for 72 hours at 30°C with pure glucose-xylose enriched with a solution of sodium phosphate potassium and ammonium sulphate. During the fermentation reaction, pyruvate is formed as an intermediary from the reduction of glucose in the glycolysis reaction, which in turn forms acetaldehyde and carbon dioxide. The acetaldehyde is further reduced to form the desired product, ethanol.
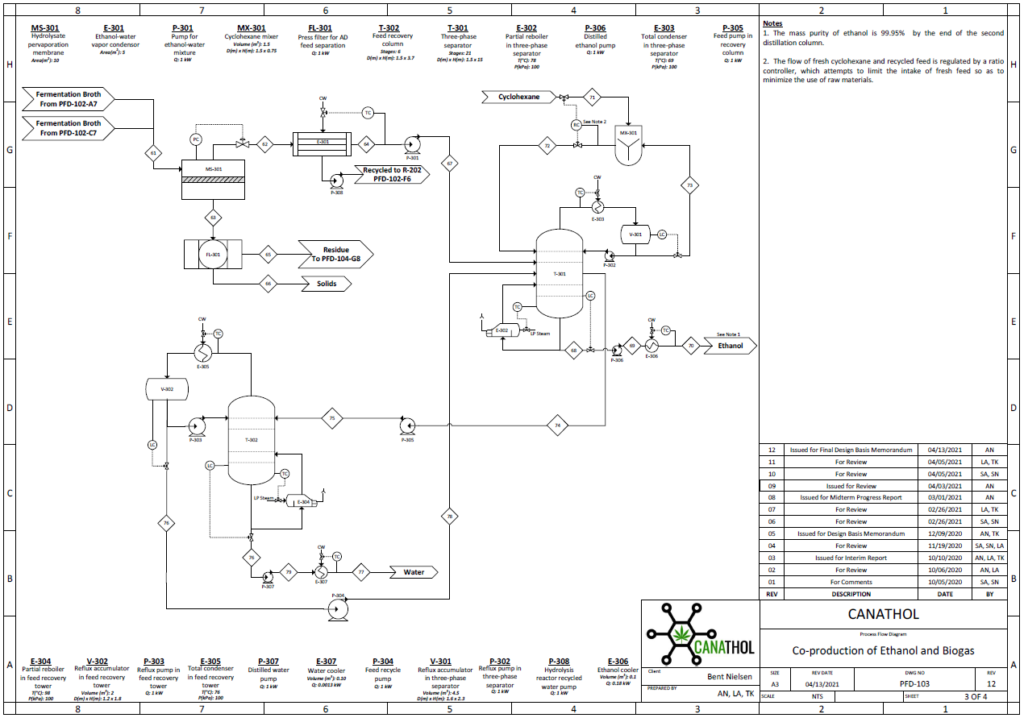
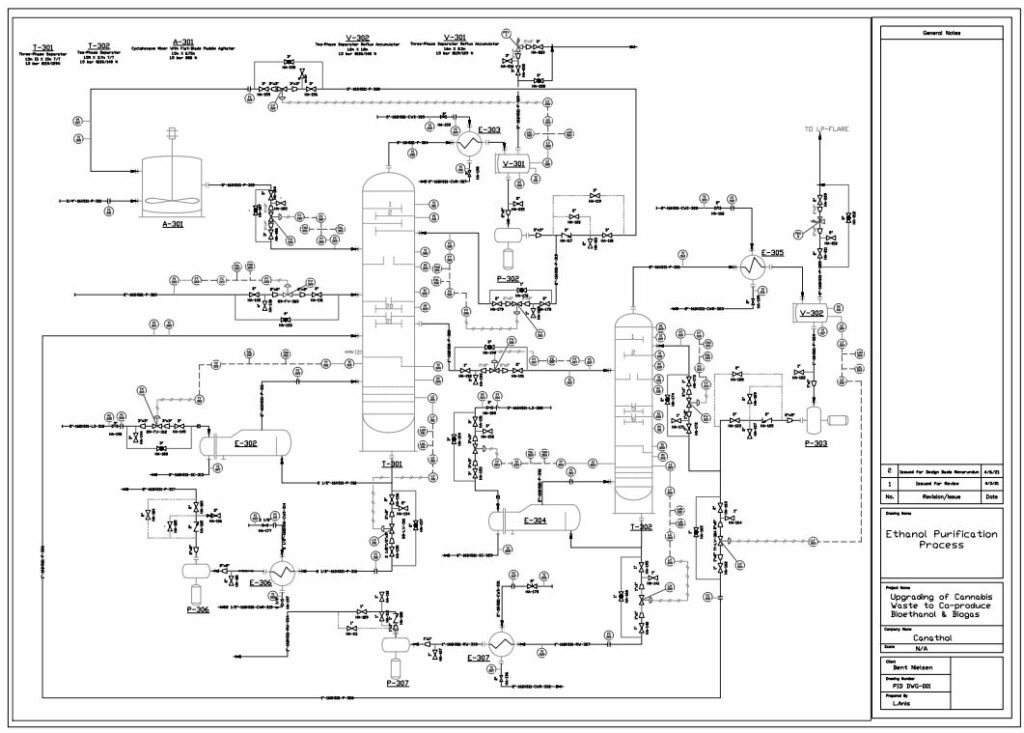
Distillation
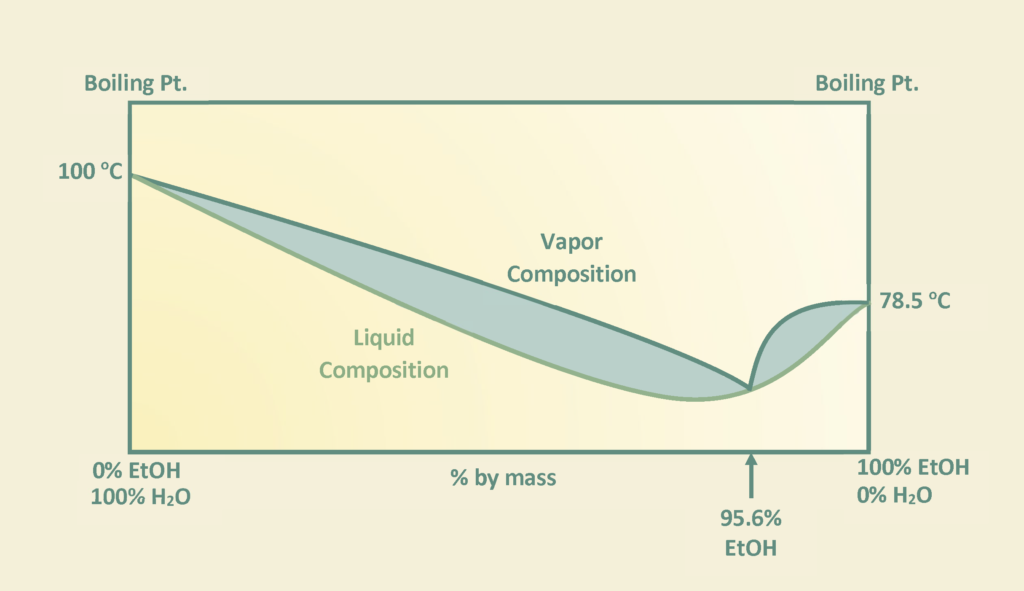
Bioethanol is a biodegradable source of energy which is considered an alternative fuel in the transportation industry with comparison to traditional fossil fuels. There are two types of ethanol commonly produced in industry: hydrated and anhydrous ethanol. Anhydrous ethanol can be produced by either catalytic hydration of ethylene or fermentation of agricultural biomass containing sugars, starch and cellulose. Canathol aims to produce anhydrous ethanol with a purity greater than 99%.
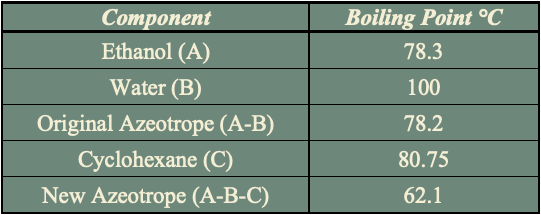
The stream post pervaporation process contains a pure binary mixture of 90% ethanol and 10% water. This binary mixture contains an azeotrope at a boiling point of 78.2℃ with an ethanol composition of 95.6% by weight at atmospheric pressure as depicted in the figure and cannot be purified any further by conventional or fractional distillation.
Therefore, other special techniques of separation were explored to break this azeotrope for the manufacturing of anhydrous ethanol with a high purity. There are various methods such as extractive distillation, azeotropic distillation, chemical dehydration, pressure swing distillation, and membrane processes to achieve anhydrous ethanol. However, extensive research was conducted for methods commonly used in industries to achieve the desired quality of anhydrous ethanol before selecting azeotropic distillation with the addition of an entrainer.
The addition of cyclohexane changes the molecular interactions between water and ethanol altering the activity coefficients and volatility of the two components. As shown in the table, cyclohexane creates a new boiling point that is lower than the boiling point of ethanol and water mixture enabling the azeotrope to be broken.
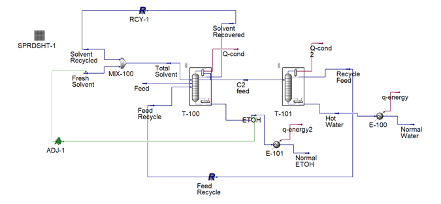
Anhydrous ethanol is produced using cyclohexane as an entrainer utilizing a setup of two distillation columns shown in the figure on the left.
The binary mixture of 90% ethanol by weight is fed at 4.3 kg/h into the three-phase separator with another stream of cyclohexane at a flowrate of 20 kg/h. A three-phase separator is utilized as the first column to ensure phase separation of the two liquid phases. Ethanol is produced as the bottoms product with a flowrate of 3.8 kg/h with a purity of 99.95%. The distillate of the three-phase separator contains a rich entrainer which is recovered and recycled back into the mixer with small amount of fresh cyclohexane entering the system to maintain the entrainer to feed ratio. Recycling the entrainer back into the system decreases the overall cost of the entrainer.
A second liquid stream, rich in ethanol exits the three-phase separator and enters the recovery conventional distillation column at a flowrate of 1.3 kg/h. The recovery column recycles the distillate back to column 1 as feed to increase the production of ethanol while water is produced as the bottoms product.
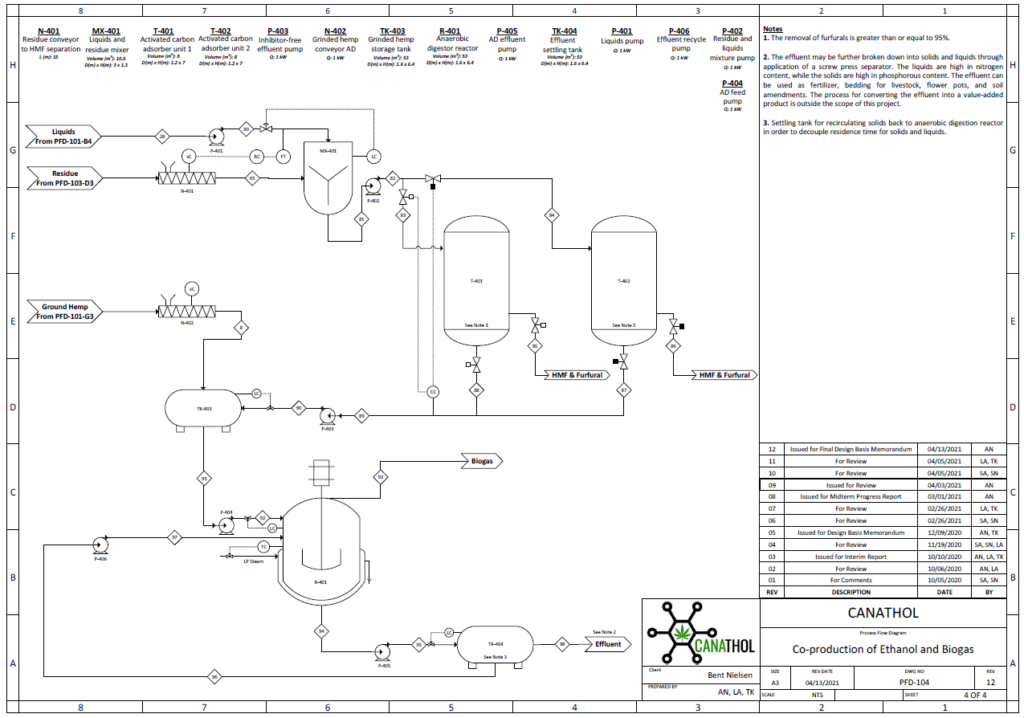
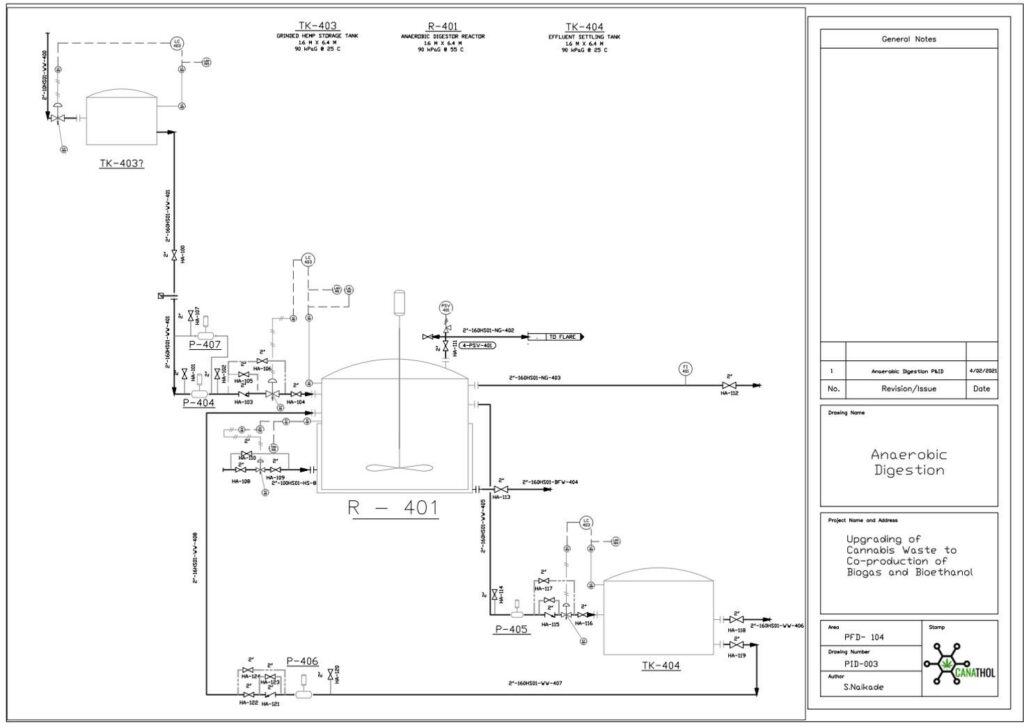
Anaerobic Digestion

The purpose of the anaerobic digestion process is to use organic waste and turn it into usable biogas. In this case, the waste enters the digester in two forms: i) chopped up hemp hurds and ii) effluent from HMF and furfural separation. Once the substrates are fed to the digester there are four crucial reactions involved in the production of biogas. These reactions are (i) hydrolysis (rate limiting step), (ii) acidogenesis, (iii) acetogenesis, and (iv) methanogenesis. The most valuable stream in the biogas is the constituent of methane, other major constituents include carbon dioxide, water vapour, and hydrogen sulfide.
Important design parameters in anaerobic digestion include total solids (TS), volatile solids (VS, biodegradable content of TS), organic loading rate (OLR, rate at which VS fed into reactor), and carbon to nitrogen ratio (C:N).
For this semester, several design parameters were modified based on the new raw feed rate to the plant. The updated feed to the digester contains 14% TS and 10% VS and consists of 2 % cellulose, 0.5% hemicellulose, 4.5% lignin, 2.2% glucose, 0.8% xylose, and 88% water. The reactor has a OLR of 4 kg VS/m3 day with a C/N ratio of 80:1. This process is modelled by a continuous fed single CSTR with a mass flowrate of (40.35) kg/h operating at 55°C and 1 atm and was simulated in Aspen Plus using the industry standard ADM1 model.
The feed of 40.35 kg/h resulted in 0.59 kg/h of biogas and 39.75 kg/h of effluent with a calculated digester volume of 53 m3 including headspace. Based on the design parameters for the reactor the biogas stream yielded a mass composition of 50.84 %of methane which is in line with typical industry values which range from 50-57% mass composition. This was a key achievement as last semester our methane mass composition percentage was only 8.14%. The reason why we were able to achieve higher methane composition is primarily due to a significantly lower plant raw feed rate enabling the selection of a more desirable OLR of 4 kg VS/m3 day which after running multiple simulations was seen to yield the highest methane composition. Previously the OLR was 10,072 kg VS/m3 day due to a high plant raw feed rate leading to the acidification of the reactor, inhibiting the methanogenic bacteria from producing methane.
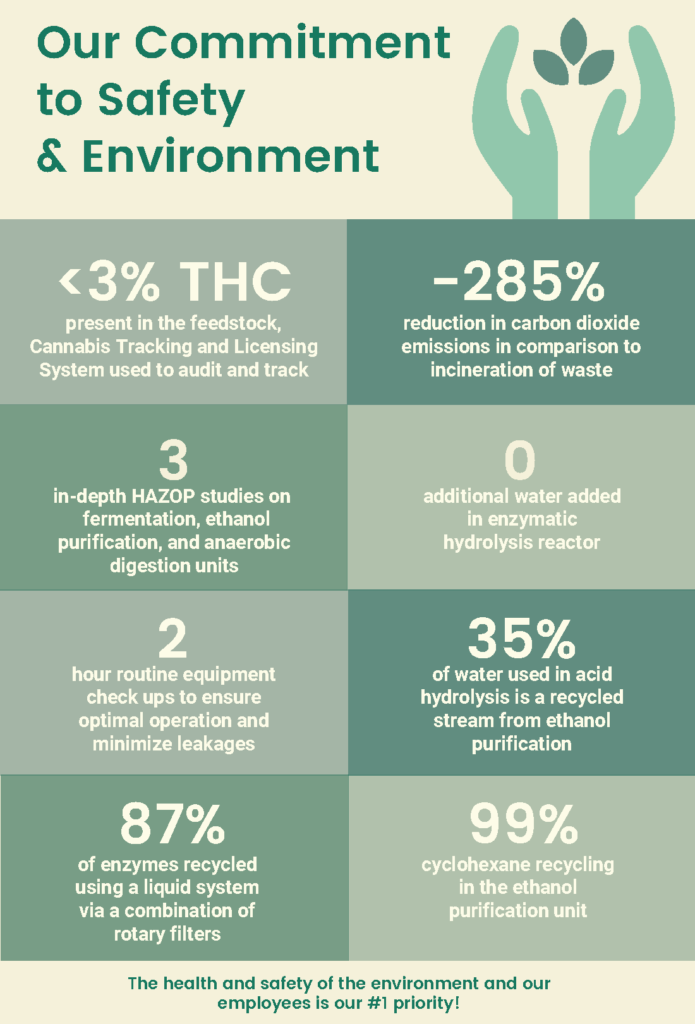
COMMITMENT TO SAFETY & ENVIRONMENT
The current Albertan Government is placing a significant emphasis on diversifying the economy, our process would help in that cause by producing renewable fuels, bioethanol, and biogas. These products will help transition the provincial economy from being predominantly hydrocarbon based to creating sustainable alternatives. Aside from diversifying the economy, our products will also help Alberta change its perception as a significant polluter and producer of carbon intensive heavy oil to a more progressive and environmentally conscious energy provider.
Since our feedstock is primarily hemp hurds which does contain THC, Health Canada requires stringent tracking and reporting during transportation of these hurds. A formal reporting system exists to aid in the auditing of the hemp hurds known as the Cannabis Tracking and Licensing System. Canathol would be required to verify the quantity of hemp hurds received from the cultivators to ensure all mass is accounted for.
One of the most significant environmental concerns during bioethanol and biogas production comes from the bacteria, enzymes, and yeast used during both bioethanol and biogas production. Using these micro-organisms introduces the risk of releasing them into the environment. However, it should be noted that since Canathol’s process uses a very small number of cultures, upon a brief analysis it can be concluded that in the event of a loss of primary containment, the culture concentration would be small and localized and hence remedial action can be taken promptly.
A hazard and operability analysis has also been conducted by Canathol on the major units of our process, highlighting key areas of improvements and safety measures to be implemented while accounting for several deviations in the process. Canathol has since integrated those recommendations into the process, keeping the risk to workers and the environment to as low as possible.
PLANT LOCATION
Canathol performed a Kepner-Tregoe analysis to determine the best location for the facility. One of the major reasons Red Deer, AB was chosen as the location was to provide the province with more jobs and to help diversify Alberta’s economy.
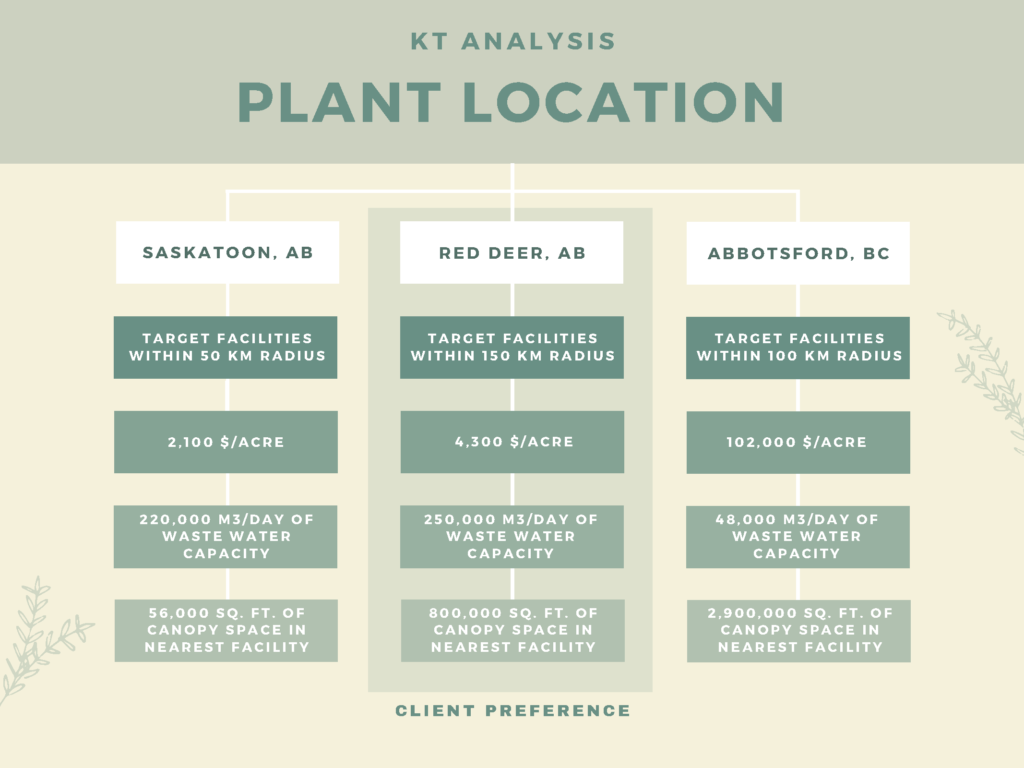
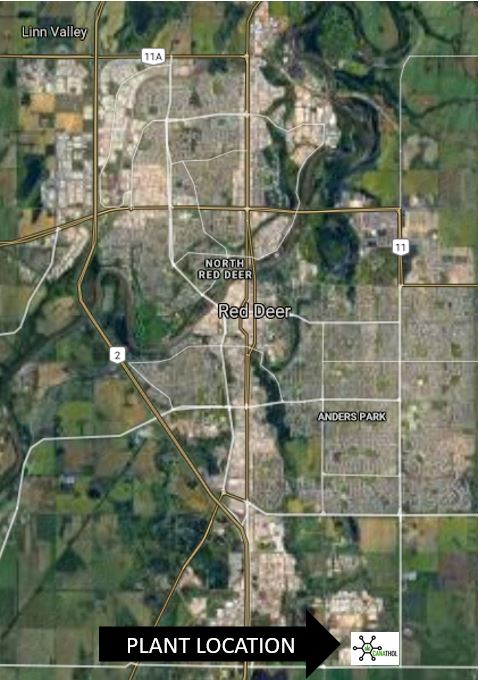
Canathol proposes a site on the corner of Range Rd 272 and Township Rd 374 to be the potential location of Canathol’s Facility. Located in Red Deer’s industrial park, a facility like this fits right into the local area.
The Team

Steven Arcus
Project Manager
Steven is in his final year of Chemical Engineering. He is a technically driven and motivated engineering student with targeted experience identifying and implementing practical, innovative, cost-effective, and safety-compliant solutions. Steven completed his internship at TC Energy with the Welding & Materials Engineering Team. Steven is a results-driven team player with a successful track record of forging strong relationships with multi-disciplinary teams to support constructive, productive, and positive interactions. Steven has an entrepreneurial mindset which is reflected on the numerous entrepreneurial classes and endeavours he has pursued over his degree. When he is not focusing on engineering, he is delving into the world of entrepreneurship.

Lamiya Anis
Separation SME
Lamiya is in her final year of Chemical Engineering and has been involved in numerous clubs in the University. Her passion lies in the oil and gas industry and renewable energy. She has completed a 16-month internship at ATCO Gas as a Pipeline Integrity and Cathodic Protection Engineering Intern where she had the opportunity to explore her interests in the energy and utility industry. Lamiya is skilled in chemical engineering design and drawings and has gained significant knowledge in AutoCAD through this project. Outside of Engineering, she enjoys travelling, trying out new foods, spending time with family and friends and playing board/card games.

Shantanu Naikade
Anaerobic Digestion SME
Shantanu is in his final year of chemical engineering and has completed a 16-month internship with Shell in the capacity of Production Engineering Intern. During his time in university Shantanu has held leadership roles on numerous clubs on campus and in industry as well as helping cofound one of his own. When he’s not studying or in a club meeting Shantanu can be found on the slopes of mountains, hiking or skiing, or on lakes, boating or skating, as well as spending time with his family and friends. He also hopes to fulfil his dream of getting his pilot’s license one day.

Ayesha Nawaz
Enzymatic Hydrolysis SME
Ayesha is a fifth year Chemical Engineering student with a background in Energy and Environment. She has a year of oil and gas industry experience at Husky Energy in the Pipeline Integrity department and past research experience working with the University’s SHARP Research Consortium. She contributed towards the project by developing a detailed design for the enzymatic hydrolysis reactor, lead the development of the PFD, performed heat integration via pinch analysis, and a HAZOP analysis on the ethanol purification unit. When she’s not working on capstone, she enjoys spending her free time hiking, freelance coding, and learning about new discoveries in the computer science field.

Talha Khan
Fermentation SME
Talha is a final year Chemical Engineering student and has completed a 12-month internship at Suncor Energy as a Health and Safety Intern where he gained valuable insights about the operation of a plant. Throughout the course of this project, Talha has had the opportunity to simulate reactions in MATLAB and complete drawings in AutoCAD, two of the many skills he acquired this past year. In addition to this project, Talha has been working as an undergraduate research intern in the geology department where he has been exposed to many common analysis techniques used in the industry. His passion for coding also coalesced this year as a side project which included data and sentiment analysis on various conversations. When he’s not writing reports or in the lab, Talha enjoys spending time with family and friends, and enjoying the good weather outside whenever possible.
Partners & Mentors
Canathol would like to thank everyone who has helped us achieve so much the past 8 months. We would especially like to thank our client Mr. Bent Nielsen (P.Eng. M.Sc.), our academic supervisor Dr. Hector De la Hoz Siegler (PhD in Chemical Engineering), and finally our Teaching Assistant Gomes Camacho Felipe (PhD Candidate).
Below are some quotes and testimonials from our mentors.
“I love to mentor students, to give them a project and see how their minds work as they tackle the understanding of the problem and then work to find a solution. In providing a small amount of guidance, I can see new ideas and skills being formed. That is what mentoring is about. Best profession ever.“
Bent Nielsen (P.Eng. M.Sc.) – Canathol’s Client/Supervisor
“In the words of Tim Notke “Hard work beats talent if talent doesn’t work hard”, Canathol’s team was able to combine both talent and hard work to overcome any challenge thrown their way.“
Gomes Camacho Felipe – ENCH 531 Teaching Assistant
Example Documents from Canathol
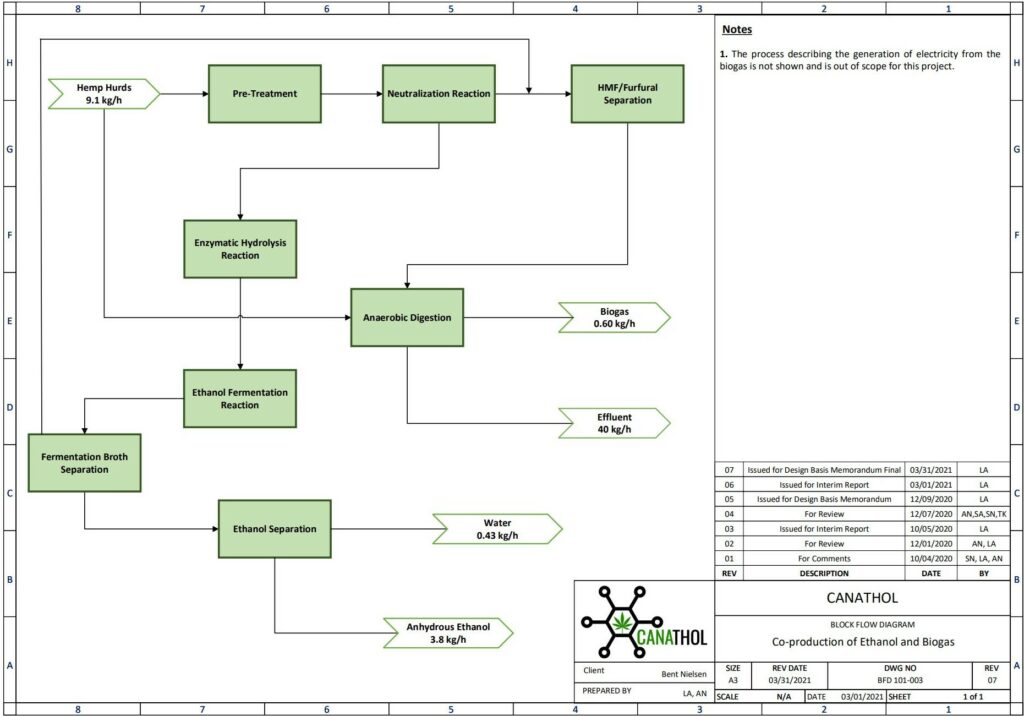
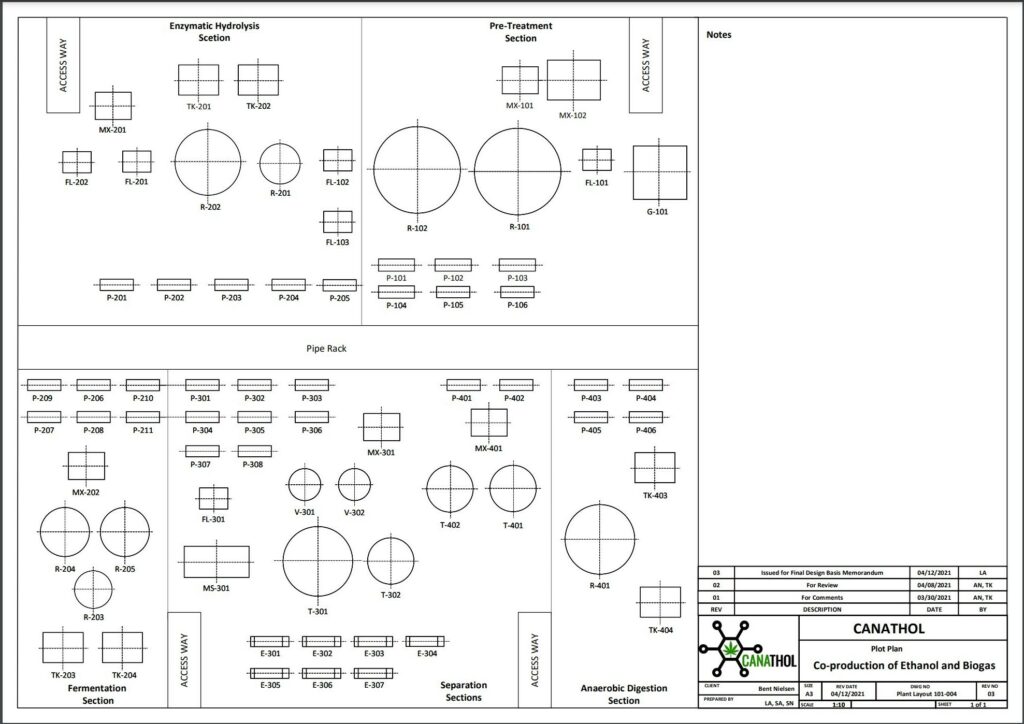
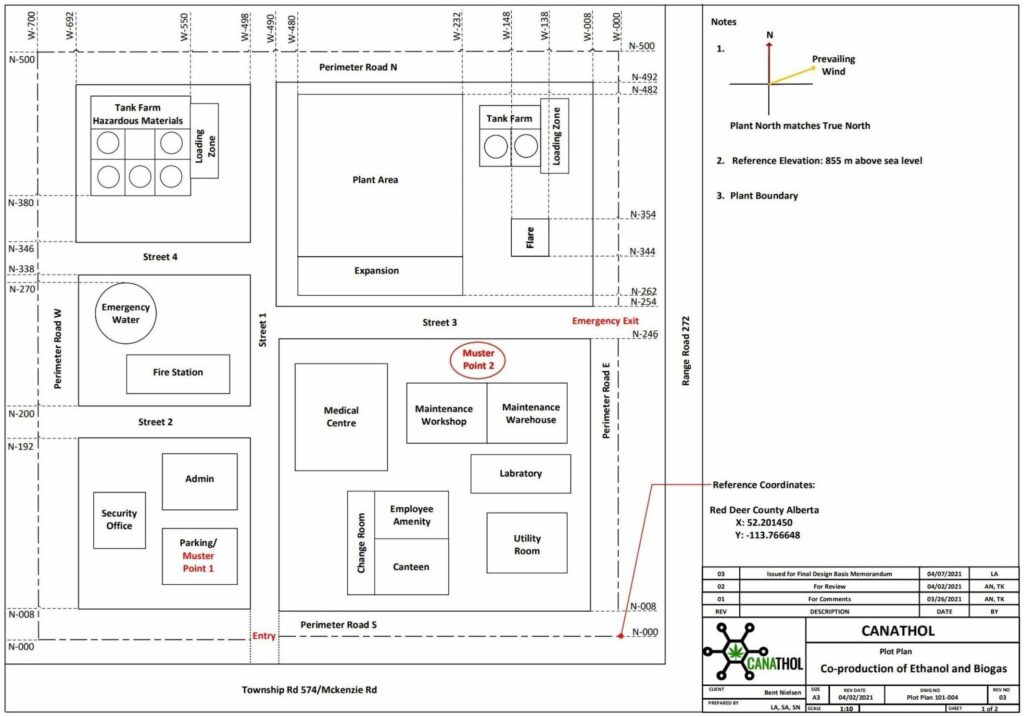
REFERENCES
Images used were created via Visio, Canva and BioRender.